Diabetes is causally associated with increased breast cancer mortality by inducing FIBCD1 to activate MCM5-mediated cell cycle arrest via modulating H3K27ac
- PMID: 40695783
- PMCID: PMC12283923
- DOI: 10.1038/s41419-025-07849-w
Diabetes is causally associated with increased breast cancer mortality by inducing FIBCD1 to activate MCM5-mediated cell cycle arrest via modulating H3K27ac
Abstract
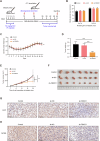
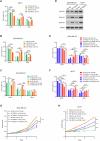
Breast cancer (BC) is the most common tumor worldwide and it has been recognized that up to one third of BC patients have co-existing diabetes mellitus (DM) (BC-DM). Although many observational studies have indicated an association between DM and BC, the causal relationship of DM and BC prognosis remained uncertain and the molecular mechanisms underlying BC-DM are largely unclear. In this study, we used causal inference methods, including g-computation (GC), inverse probability of treatment weighting (IPTW), targeted maximum likelihood estimation (TMLE), and TMLE-super learner (TMLE-SL), to comprehensively analyze the association of DM with BC mortality in a cohort of 3386 BC patients. We found that the adjusted odds ratios (OR) and 95% confidence intervals (95% CI) for 5-year mortality in BC-DM patients were 1.926 (1.082, 2.943), 2.268 (1.063, 3.974), 1.917 (1.091, 2.953), and 2.113 (1.365, 3.270), respectively. Further transcriptomic and qPCR analyses identified that FIBCD1 was highly expressed in BC-DM tumor tissues and in BC cells under hyperglycemia conditions. Functionally, upregulation of FIBCD1 promoted proliferation, migration, and invasion capacities of BC cells in a glucose level-dependent manner. While knockdown of FIBCD1 suppressed BC tumor growth in diabetic mice. Integrated RNA-seq and Ribo-seq analysis revealed that MCM5 was a target of FIBCD1. Mechanistically, hyperglycemia-activated FIBCD1 promoted MCM5 expression to induce S-phase cell cycle arrest by upregulating histone H3K27ac levels in MCM5 promoter via the PDH-acetyl-CoA axis. Our findings provide new evidence that co-existing DM has a causal effect on overall mortality in BC-DM patients. Targeting FIBCD1 may be a promising therapy for BC-DM.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval: Human sample collection and cohort study were approved by the Clinical Research Ethics Committee of Shenzhen University (Approved No. 2019015). Animal experiments were performed in accordance with the guidelines approved by the Institutional Animal Care and Use Committee of Shenzhen University Medical School (Approved No. IACUC-202300060).
Figures


Similar articles
-
Upregulated SAE1 Drives Tumorigenesis and Is Associated with Poor Clinical Outcomes in Breast Cancer.Breast J. 2024 Jun 30;2024:2981722. doi: 10.1155/2024/2981722. eCollection 2024. Breast J. 2024. PMID: 39742381 Free PMC article.
-
circACTN4 promotes breast cancer cell cycle progression and oncogenesis via c-MYC induced histone H4 acetylation.Oncol Res. 2025 Jun 26;33(7):1709-1722. doi: 10.32604/or.2025.061721. eCollection 2025. Oncol Res. 2025. PMID: 40612865 Free PMC article.
-
Systematic review on urine albumin testing for early detection of diabetic complications.Health Technol Assess. 2005 Aug;9(30):iii-vi, xiii-163. doi: 10.3310/hta9300. Health Technol Assess. 2005. PMID: 16095545
-
A lipid metabolism-related gene signature for risk stratification and prognosis prediction in patients with breast cancer.Sci Rep. 2025 Jul 26;15(1):27281. doi: 10.1038/s41598-025-10879-1. Sci Rep. 2025. PMID: 40715365 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. - PubMed
-
- Yancik R, Wesley MN, Ries LA, Havlik RJ, Edwards BK, Yates JW. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285:885–92. - PubMed
-
- Tammemagi CM, Nerenz D, Neslund-Dudas C, Feldkamp C, Nathanson D. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA. 2005;294:1765–72. - PubMed
MeSH terms
Substances
Grants and funding
- 41977372/National Natural Science Foundation of China (National Science Foundation of China)
- 20231120193941001/Shenzhen Science and Technology Innovation Commission
- 20231121155815002/Shenzhen Science and Technology Innovation Commission
- 20231120124911001/Shenzhen Science and Technology Innovation Commission
- 2024A1515012760/Natural Science Foundation of Guangdong Province (Guangdong Natural Science Foundation)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

