Accelerated brain ageing during the COVID-19 pandemic
- PMID: 40695794
- PMCID: PMC12284169
- DOI: 10.1038/s41467-025-61033-4
Accelerated brain ageing during the COVID-19 pandemic
Abstract
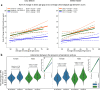
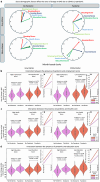
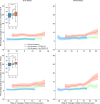
The impact of SARS-CoV-2 and the COVID-19 pandemic on brain health is recognised, yet specific effects remain understudied. We investigate the pandemic's impact on brain ageing using longitudinal neuroimaging data from the UK Biobank. Brain age prediction models are trained from hundreds of multi-modal imaging features using a cohort of 15,334 healthy participants. These models are then applied to an independent cohort of 996 healthy participants with two magnetic resonance imaging scans: either both collected before the pandemic (Control groups), or one before and one after the pandemic onset (Pandemic group). Our findings reveal that, even with initially matched brain age gaps (predicted brain age vs. chronological age) and matched for a range of health markers, the pandemic significantly accelerates brain ageing. The Pandemic group shows on average 5.5-month higher deviation of brain age gap at the second time point compared with controls. Accelerated brain ageing is more pronounced in males and those from deprived socio-demographic backgrounds and these deviations exist regardless of SARS-CoV-2 infection. However, accelerated brain ageing correlates with reduced cognitive performance only in COVID-infected participants. Our study highlights the pandemic's significant impact on brain health, beyond direct infection effects, emphasising the need to consider broader social and health inequalities.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: R.G.J. reports research grants or contracts from AstraZeneca, Galecto, GlaxoSmithKline, Nordic Bioscience, Redx and Pliant, with all payments made to his institution. He has served as a consultant for AbbVie, AdAlta, Apollo Therapeutics, Arda Therapeutics, AstraZeneca, Brainomix, Bristol Myers Squibb, Chiesi, Cohbar, Galecto, GlaxoSmithKline, Mediar Therapeutics, Redx, Syndax and Pliant. He has received honoraria for lectures, presentations, speaker bureau participation, manuscript writing, or educational events from Boehringer Ingelheim, Chiesi, Roche and AstraZeneca. He has received payment for expert testimony from Pinsent Masons LLP. He has served on data safety monitoring boards or advisory boards for Boehringer Ingelheim, Galapagos and Vicore. He holds an unpaid advisory board role at NuMedii and serves as President of Action for Pulmonary Fibrosis. He is also Chair of the Editorial Board of BMJ Open Respiratory Research. All other authors declare no competing interests.
Figures


Similar articles
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2022 Jul 22;7(7):CD013705. doi: 10.1002/14651858.CD013705.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866452 Free PMC article.
-
Measures implemented in the school setting to contain the COVID-19 pandemic.Cochrane Database Syst Rev. 2022 Jan 17;1(1):CD015029. doi: 10.1002/14651858.CD015029. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 May 2;5:CD015029. doi: 10.1002/14651858.CD015029.pub2. PMID: 35037252 Free PMC article. Updated.
-
Service Interruption in HIV Care Amid COVID-19 Pandemic in Myanmar: Results From Analysis of Routine Program Data 2018-2022.J Int Assoc Provid AIDS Care. 2024 Jan-Dec;23:23259582241299466. doi: 10.1177/23259582241299466. J Int Assoc Provid AIDS Care. 2024. PMID: 39648608 Free PMC article.
-
Effects of city design on transport mode choice and exposure to health risks during and after a crisis: a retrospective observational analysis.Lancet Planet Health. 2025 Jun;9(6):e467-e479. doi: 10.1016/S2542-5196(25)00088-9. Lancet Planet Health. 2025. PMID: 40516538
Cited by
-
Mental Health and Cognitive Outcomes in Patients Six Months After Testing Positive Compared with Matched Patients Testing Negative for COVID-19 in a Non-Hospitalized Sample: A Matched Retrospective Cohort Study.Int J Environ Res Public Health. 2025 Aug 9;22(8):1249. doi: 10.3390/ijerph22081249. Int J Environ Res Public Health. 2025. PMID: 40869835 Free PMC article.
References
-
- Mavrikaki, M., Lee, J. D., Solomon, I. H. & Slack, F. J. Severe COVID-19 is associated with molecular signatures of aging in the human brain. Nat. Aging2, 1130–1137 (2022). - PubMed
MeSH terms
Grants and funding
- MR/W014491/1/RCUK | Medical Research Council (MRC)
- MR/V005324/1/RCUK | Medical Research Council (MRC)
- MR/W014491/1/RCUK | Medical Research Council (MRC)
- MR/V005324/1/RCUK | Medical Research Council (MRC)
- MR/W014491/1/RCUK | Medical Research Council (MRC)
- MR/V005324/1/RCUK | Medical Research Council (MRC)
- MR/W014491/1/RCUK | Medical Research Council (MRC)
- MR/V005324/1/RCUK | Medical Research Council (MRC)
- Nottingham Biomedical Research Centre/DH | National Institute for Health Research (NIHR)
- Nottingham Biomedical Research Centre/DH | National Institute for Health Research (NIHR)
- Nottingham Biomedical Research Centre/DH | National Institute for Health Research (NIHR)
- Nottingham Biomedical Research Centre/DH | National Institute for Health Research (NIHR)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

