Genotype B3.13 influenza A(H5N1) viruses isolated from dairy cattle demonstrate high virulence in laboratory models, but retain avian virus-like properties
- PMID: 40695800
- PMCID: PMC12284218
- DOI: 10.1038/s41467-025-61757-3
Genotype B3.13 influenza A(H5N1) viruses isolated from dairy cattle demonstrate high virulence in laboratory models, but retain avian virus-like properties
Abstract
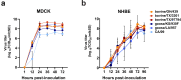
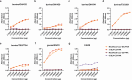
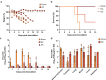
In March 2024, clade 2.3.4.4b highly pathogenic avian influenza A(H5N1) viruses were first detected in U.S. dairy cattle. Similar viruses have since caused 70 zoonotic human infections. To assess changes to zoonotic potential, we characterized A(H5N1) clade 2.3.4.4b viruses isolated from cows' milk and birds. Bovine-derived viruses are lethal in mice and ferrets and transmit to direct but not airborne contact ferrets. All viruses replicate in human bronchial epithelial cells despite preferentially binding avian virus-like receptors. The bovine-derived viruses remain susceptible to FDA-approved antivirals, and they are inhibited by sera from ferrets vaccinated with WHO-recommended candidate vaccine viruses (CVV) or human sera from clade 2.3.4.4c vaccinees. While 2.3.4.4b viruses induce severe disease in mammalian models, they retain many avian virus-like characteristics. Combined, we conclude that the risk of contemporary bovine-derived viruses to humans not in contact with affected animals is low. However, heightened vigilance remains essential to promptly detect and respond to any changes.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- WHO. Avian Influenza Weekly Update # 990: 21 March 2025. 4 (2025).
-
- WHO. Global Influenza Program, (https://www.who.int/publications/m/item/cumulative-number-of-confirmed-h..., 2024).
-
- CDC. H5N1 Bird Flu Detections across the United States in Backyard and Commercial Poultry, https://www.cdc.gov/bird-flu/situation-summary/data-map-commercial.html (2024).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

