Hamsters with long COVID present distinct transcriptomic profiles associated with neurodegenerative processes in brainstem
- PMID: 40695836
- PMCID: PMC12283958
- DOI: 10.1038/s41467-025-62048-7
Hamsters with long COVID present distinct transcriptomic profiles associated with neurodegenerative processes in brainstem
Abstract
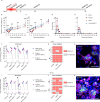
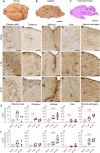
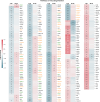
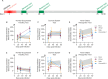
Following infection with SARS-CoV-2, patients may experience with one or more symptoms that appear or persist over time. Neurological symptoms associated with long COVID include anxiety, depression, and memory impairment. However, the exact underlying mechanisms are not yet fully understood. Using golden hamsters as a model, we provide further evidence that SARS-CoV-2 is neuroinvasive and can persistently infect the brain, as viral RNA and replicative virus are detected in the brainstem 80 days after the initial infection. Infected hamsters exhibit a neurodegenerative signature in the brainstem, characterized by overexpression of innate immunity genes, and altered expression of genes involved in the dopaminergic and glutamatergic synapses, in energy metabolism, and in proteostasis. These infected animals exhibit persistent depression-like behavior, impaired short-term memory, and late-onset signs of anxiety. Finally, we provide evidence that viral and immunometabolic mechanisms coexist in the brainstem of SARS-CoV-2-infected hamsters, contributing to the manifestation of neuropsychiatric and cognitive symptoms.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
-
- COVID-19 cases | WHO COVID-19 dashboard. datadothttps://data.who.int/dashboards/covid19/cases.
-
- Salmon, D. et al. Patients with Long COVID continue to experience significant symptoms at 12 months and factors associated with improvement: A prospective cohort study in France (PERSICOR). Int. J. Infect. Dis.140, 9–16 (2024). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

