Exploring thoracic aorta ECM alterations in Marfan syndrome: insights into aorta wall structure
- PMID: 40695874
- PMCID: PMC12284111
- DOI: 10.1038/s41598-025-09665-w
Exploring thoracic aorta ECM alterations in Marfan syndrome: insights into aorta wall structure
Abstract
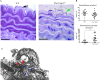
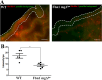
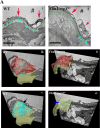
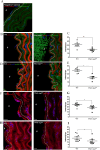
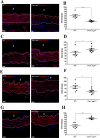
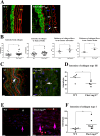
Marfan syndrome is a connective tissue disorder caused by FBN1 mutations, leading to aortic wall fragility and increased susceptibility to aneurysm and dissection. This study investigated microstructural and molecular alterations in the thoracic aorta of Fbn1mgΔlpn mice, with a focus on the tunica intima and media. Histological and ultrastructural analyses demonstrated elastic fiber fragmentation and reduced fibrillin-1 expression. In the intima, endothelial cells showed partial detachment and decreased levels of fibrillin-1, perlecan, collagen IV, and α5β1 integrins, suggesting compromised adhesion to the extracellular matrix. Serial block-face scanning electron microscopy revealed discontinuities in the internal elastic lamina. In the media, we observed reduced fibronectin, altered α5β1 integrin distribution, and increased α-smooth muscle actin, indicative of remodeling in elastin-contractile units. Second harmonic generation imaging revealed increased collagen deposition, and thickness in areas of elastic fiber disruption, along with reduced and disorganized type III collagen and increased type I collagen. Echocardiographic evaluation showed aortic root, and ascendant-aorta dilatation, altered blood flow, and diastolic dysfunction. Elastic fiber integrity correlated strongly with fibrillin-1 expression (r = 0.93, p = 0.0003) and aortic blood flow (r = 0.77, p = 0.0064). These results suggest that early alterations in matrix organization and endothelial-matrix interactions may contribute to aortic wall weakening in Fbn1mgΔlpn mice.
Keywords: Aneurysm; Aorta; Collagen; Extracellular matrix; Fibrillin-1; Marfan syndrome.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval: All experiments were carried out in accordance with the relevant guidelines.
Figures

References
-
- Souza, R. B. et al. Critical analysis of aortic dysmorphism in Marfan syndrome. J. Morphological Sci.34 (1), 1–6. 10.4322/jms.102516 (2017). - DOI
-
- Milewicz, D. M. et al. Marfan syndrome. Nat. Rev. Dis. Prim.7(1), 64. https://doi.org/10.1038/s41572-021-00298-7 (2021). - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 001/Coordenação de Aperfeiçoamento de Pessoal de Nível Superior
- 001/Coordenação de Aperfeiçoamento de Pessoal de Nível Superior
- 88887.899440/2023-00/Coordenação de Aperfeiçoamento de Pessoal de Nível Superior
- 001/Coordenação de Aperfeiçoamento de Pessoal de Nível Superior
- 001/Coordenação de Aperfeiçoamento de Pessoal de Nível Superior
- 001/Coordenação de Aperfeiçoamento de Pessoal de Nível Superior
- 001/Coordenação de Aperfeiçoamento de Pessoal de Nível Superior
- 001/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 001/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 001/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 001/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 001/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 397484323 [TRR259/B09]/Deutsche Forschungsgemeinschaft
- 397484323 [TRR259/B09]/Deutsche Forschungsgemeinschaft
- 2013/07937-8/CEPID Redoxoma Network, supported by Fundação de Amparo à Pesquisa do Estado de São Paulo
- Gada Canada Aortic Research Grant Award/Genetic Aortic Disorders Association Canada
- MR/S037829/1/MRC_/Medical Research Council/United Kingdom
- MR/S037829/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Medical

