Shear stress-triggered adenosine triphosphate regulates angiogenic properties of human periodontal ligament stem cells
- PMID: 40695923
- PMCID: PMC12284249
- DOI: 10.1038/s41598-025-12323-w
Shear stress-triggered adenosine triphosphate regulates angiogenic properties of human periodontal ligament stem cells
Abstract
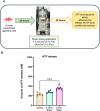
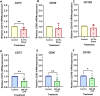
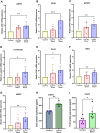
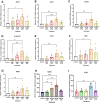
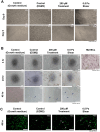
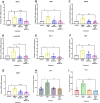
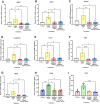
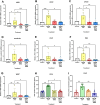
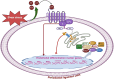
Mechanical forces stimulate human periodontal ligament stem cells (HPDLSCs) to release extracellular adenosine triphosphate (eATP). The eATP impacts various functions of HPDLSCs, i.e., immunosuppression and inflammation. eATP has been reported to promote the angiogenesis of pulmonary vascular endothelial cells. Shear force, one of the mechanical forces involved in orthodontic tooth movement, influences osteogenic differentiation and ECM remodeling of HPDLSCs. However, the relationship between shear force and the impact of eATP on endothelial differentiation and angiogenic characteristics of HPDLSCs remains unclear. This study aimed to determine the response of HPDLSCs on endothelial differentiation and angiogenic properties after shear stress loading and eATP treatment as well as explored the mechanism of eATP-involved in angiogenic responses of HPDLSCs. Shear stress application at 5 dyn/cm2 for 24 h stimulated the release of ATP by HPDLSCs. Both shear stress and 200 µM eATP promoted the expression of endothelial differentiation and angiogenic markers (ANG1, CD31, EPCR, e-selectin, FLK1, TIE2, VEGF). Blockade of specific P2Y1 receptor and intracellular calcium signaling attenuated eATP-induced expression of endothelial differentiation and angiogenic expression. Both shear stress and eATP have stimulatory effects on endothelial differentiation and angiogenesis in HPDLSCs through P2Y1-intracellular calcium signaling.
Keywords: Angiogenesis; Endothelial differentiation; Extracellular ATP; Human periodontal ligament stem cells; Shear stress.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
References
-
- Kanjanamekanant, K., Luckprom, P. & Pavasant, P. Mechanical stress-induced interleukin-1beta expression through adenosine triphosphate/P2X7 receptor activation in human periodontal ligament cells. J. Periodontal Res.48, 169–176. 10.1111/j.1600-0765.2012.01517.x (2013). - PubMed
-
- Luckprom, P., Kanjanamekanant, K. & Pavasant, P. Role of connexin43 hemichannels in mechanical stress-induced ATP release in human periodontal ligament cells. J. Periodontal Res.46, 607–615. 10.1111/j.1600-0765.2011.01379.x (2011). - PubMed
-
- Takahara, N. et al. Real-time imaging of ATP release induced by mechanical stretch in human airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol.51, 772–782. 10.1165/rcmb.2014-0008OC (2014). - PubMed
MeSH terms
Substances
Grants and funding
- B16F640118/the National Science, Research, and Innovative Fund (NSRF), Thailand, via the Program Management Unit for Human Resources & Institutional Development, Research and Innovation
- B16F640118/the National Science, Research, and Innovative Fund (NSRF), Thailand, via the Program Management Unit for Human Resources & Institutional Development, Research and Innovation
- HEAF67320037/the Thailand Science Research and Innovation Fund, Chulalongkorn University
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

