Bisphenol A suppresses colon epithelial cell responses via G0/G1-phase arrest, MAPK and PI3K/AKT pathway modulation, and MMP-2/9 Inhibition by upregulating p21WAF1
- PMID: 40695970
- PMCID: PMC12283940
- DOI: 10.1038/s41598-025-11700-9
Bisphenol A suppresses colon epithelial cell responses via G0/G1-phase arrest, MAPK and PI3K/AKT pathway modulation, and MMP-2/9 Inhibition by upregulating p21WAF1
Abstract
Bisphenol A (BPA) is a non-steroidal endocrine-disrupting chemical compound with applications in the production of epoxy resins and polycarbonates. Accumulating evidence suggests that BPA damages various organs and tissues, including those of the reproductive, immune, and neuroendocrine systems. However, the mechanisms by which BPA affects the intestinal tract have not been fully elucidated. We explored the adverse effects of BPA on human colonic epithelial cells in vitro by performing comprehensive viability, proliferation, invasion, and migration assays on HCT 116 and HCT-8 cells. BPA suppressed the proliferation of both cell types by regulating cell cycle progression and modulating the mitogen-activated protein kinase and phosphatidylinositol 3-kinase/protein kinase B pathways. Furthermore, BPA treatment significantly reduced the induction of matrix metalloproteinases-2 and -9 by inhibiting the binding activity of specificity protein-1, nuclear factor kappa B, and activator protein-1, thereby interfering with cell migration and invasion. This BPA-induced regulation of colonic epithelial cell proliferation, migration, invasion, and MAPK/AKT pathway was reversed by silencing p21WAF1 with siRNA. Collectively, our data indicate that BPA inhibits the proliferation and mobility of human colonic epithelial cells via p21WAF1 induction. This study provides valuable information into the precise mechanisms underlying the adverse effects of BPA on colonic epithelial cells.
Keywords: Bisphenol A; Cell cycle; Human colonic epithelial cells; Migration; Proliferation; Signaling pathway.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures




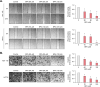


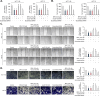
Similar articles
-
RETRACTED: Shikonin Inhibits the Migration and Invasion of Human Glioblastoma Cells by Targeting Phosphorylated β-Catenin and Phosphorylated PI3K/Akt: A Potential Mechanism for the Anti-Glioma Efficacy of a Traditional Chinese Herbal Medicine.Int J Mol Sci. 2015 Oct 9;16(10):23823-48. doi: 10.3390/ijms161023823. Int J Mol Sci. 2015. Retraction in: Int J Mol Sci. 2025 Jun 20;26(13):5922. doi: 10.3390/ijms26135922. PMID: 26473829 Free PMC article. Retracted.
-
Hesperetin Inhibits Bladder Cancer Cell Proliferation and Promotes Apoptosis and Cycle Arrest by PI3K/AKT/FoxO3a and ER Stressmitochondria Pathways.Curr Med Chem. 2025;32(19):3879-3904. doi: 10.2174/0109298673283888231217174702. Curr Med Chem. 2025. PMID: 38357946
-
Activation of G protein-coupled receptor 30 by thiodiphenol promotes proliferation of estrogen receptor α-positive breast cancer cells.Chemosphere. 2017 Feb;169:204-211. doi: 10.1016/j.chemosphere.2016.11.066. Epub 2016 Nov 20. Chemosphere. 2017. PMID: 27880919
-
A comprehensive review of medicinal plants and their beneficial roles in alleviating bisphenol A-induced organ toxicity.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jul;398(7):7801-7876. doi: 10.1007/s00210-025-03795-8. Epub 2025 Feb 11. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39932506 Review.
-
A systematic study of the release of bisphenol A by orthodontic materials and its biological effects.Int Orthod. 2016 Dec;14(4):399-417. doi: 10.1016/j.ortho.2016.10.005. Epub 2016 Nov 14. Int Orthod. 2016. PMID: 27856373
References
-
- Jun, J. H., Oh, J. E., Shim, J., Kwak, Y. & Cho, J. S. Effects of bisphenol A on the proliferation, migration, and tumor growth of colon cancer cells: In vitro and in vivo evaluation with mechanistic insights related to ERK and 5-HT3. Food Chem. Toxicol.158, 112662 (2021). - PubMed
-
- Flint, S., Markle, T., Thompson, S. & Wallace, E. Bisphenol A exposure, effects, and policy: A wildlife perspective. J. Environ. Manag.104, 19–34 (2012). - PubMed
-
- Kang, J., Kondo, F. & Katayama, Y. Human exposure to bisphenol A. Toxicology226, 79–89 (2006). - PubMed
-
- Murata, M. & Kang, J. Bisphenol A (BPA) and cell signaling pathways. Biotechnol. Adv.36, 311–327 (2018). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

