Relationship between aggregate index of systemic inflammation and mortality from CCD and malignant neoplasms in diabetic patients
- PMID: 40696035
- PMCID: PMC12283919
- DOI: 10.1038/s41598-025-12094-4
Relationship between aggregate index of systemic inflammation and mortality from CCD and malignant neoplasms in diabetic patients
Abstract
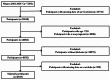
Inflammation has been recognized as a pivotal factor in the pathophysiology of diabetes. The aggregate index of systemic inflammation (AISI) has recently been proposed as a novel biomarker for evaluating inflammatory status and predicting clinical outcomes. However, evidence on the association between AISI and mortality in diabetic patients remains limited. To address this knowledge gap, we aimed to investigate the association between AISI and mortality risk from cardio-cerebrovascular disease (CCD) and malignant neoplasms in diabetic patients. We analyzed data from the National Health and Nutrition Examination Survey (NHANES, 2001-2018). Multivariable-adjusted Cox models revealed strong associations between elevated AISI levels and CCD mortality (HR 1.18, 95% CI 1.11-1.26) as well as malignant neoplasm mortality (HR 1.20, 95% CI 1.10-1.30). Kaplan-Meier analysis showed that higher AISI was associated with lower survival in diabetic patients for both CCD and malignant neoplasms. Restricted cubic spline (RCS) analysis demonstrated an increased risk of mortality from CCD and malignant neoplasms in diabetic patients with elevated AISI levels. Subgroup and sensitivity analyses confirmed the robustness of these findings. In adults with diabetes, elevated AISI levels are strongly associated with an increased risk of mortality from CCD and malignant neoplasms.
Keywords: Aggregate index of systemic inflammation (AISI); Cardio-cerebrovascular disease (CCD) mortality; Diabetes; Malignant neoplasm mortality.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics statement: Studies involving human participants were reviewed and approved by the National Health and Nutrition Examination Survey (NHNES). The patients provided written informed consent to participate in the study.
Figures


References
MeSH terms
Substances
Grants and funding
- 82060337/National Natural Science Fund
- JCYJ20220531092412028/Shenzhen City Science and Technology Plan Project Basic Research Surface Project
- wsjkkj2022057/Baotou City Health Science and Technology Project
- 2025MS08169/Natural Science Foundation of Inner Mongolia Autonomous Region
- 2024GLLH0605/Inner Mongolia Public Hospital research joint fund Science and Technology Project
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

