CXCL12 chemokine dimer signaling modulates acute myelogenous leukemia cell migration through altered receptor internalization
- PMID: 40696056
- PMCID: PMC12284246
- DOI: 10.1038/s41598-025-12005-7
CXCL12 chemokine dimer signaling modulates acute myelogenous leukemia cell migration through altered receptor internalization
Abstract
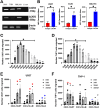
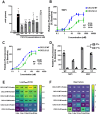
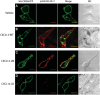
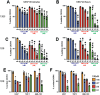
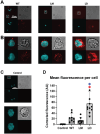
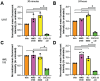
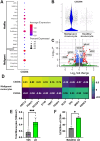
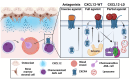
Acute myeloid leukemia (AML) is a malignancy of immature myeloid blast cells with stem-like and chemoresistant cells being retained in the bone marrow through CXCL12-CXCR4 signaling. Current CXCR4 inhibitors that mobilize AML cells into the bloodstream have failed to improve patient survival, likely reflecting persistent chemokine receptor localization on target cells. Here we characterize the signaling properties of CXCL12-locked dimer (CXCL12-LD), a bioengineered variant of the naturally occurring oligomer of CXCL12. CXCL12-LD, in contrast to wild-type or locked monomer variants, was unable to induce chemotaxis in AML cells. CXCL12-LD binding to CXCR4 decreased G protein, β-arrestin, and intracellular calcium mobilization signaling pathways, and indicated the locked dimer is a partial agonist of CXCR4. Despite these partial agonist properties, CXCL12-LD increased CXCR4 internalization compared to wild-type and monomeric CXCL12. Analysis of a previously published AML transcriptomic data showed CXCR4 positive AML cells co-express genes involved in survival, proliferation, and maintenance of a blast-like state. The CXCL12-LD partial agonist effectively mobilized stem cells into the bloodstream in mice suggesting a potential role for their use in targeting CXCR4. Together, our results suggest that enhanced internalization by CXCL12-LD partial agonist can avoid pharmacodynamic tolerance and may identify new avenues to better target GPCRs.
Keywords: Acute myeloid leukemia; CXCR4; Cell signaling; Chemokines; GPCR; Receptor internalization.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: MBD and FP have financial interests in Protein Foundry, LLC and Xlock Biosciences, Inc. The remaining authors have no conflicts to disclose.
Figures
Update of
-
CXCL12 chemokine dimer signaling modulates acute myelogenous leukemia cell migration through altered receptor internalization.bioRxiv [Preprint]. 2025 Apr 14:2024.08.26.609725. doi: 10.1101/2024.08.26.609725. bioRxiv. 2025. Update in: Sci Rep. 2025 Jul 22;15(1):26625. doi: 10.1038/s41598-025-12005-7. PMID: 39253415 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

