Combination of pembrolizumab and radiotherapy induces systemic antitumor immune responses in immunologically cold non-small cell lung cancer
- PMID: 40696153
- PMCID: PMC12559004
- DOI: 10.1038/s43018-025-01018-w
Combination of pembrolizumab and radiotherapy induces systemic antitumor immune responses in immunologically cold non-small cell lung cancer
Abstract
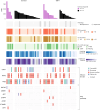
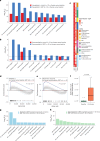
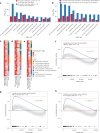
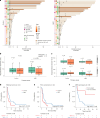
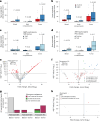
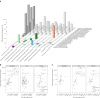
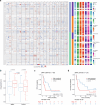
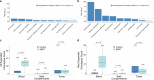
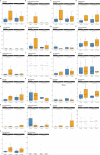
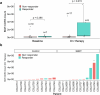
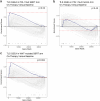
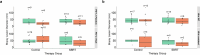
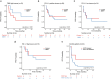
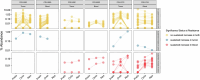
The abscopal effects of radiation may sensitize immunologically cold tumors to immune checkpoint inhibition. We investigated the immunostimulatory effects of radiotherapy leveraging multiomic analyses of serial tissue and blood biospecimens (n = 293) from a phase 2 clinical trial of stereotactic body radiation therapy (SBRT) followed by pembrolizumab in metastatic non-small cell lung cancer ( NCT02492568 ). Participants with immunologically cold tumors (low tumor mutation burden, null programmed death ligand 1 expression or Wnt pathway mutations) had significantly longer progression-free survival in the SBRT arm. Induction of interferon-γ, interferon-α and antigen processing and presentation gene sets was significantly enriched after SBRT in nonirradiated tumor sites. Significant on-therapy expansions of new and pre-existing T cell clones in both the tumor (abscopal) and the blood (systemic) compartments were noted alongside clonal neoantigen-reactive autologous T cell responses in participants with long-term survival after radioimmunotherapy. These findings support the systemic immunomodulatory and antitumor effects of radioimmunotherapy and may open a therapeutic window of opportunity to overcome immunotherapy resistance.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: V.A. receives research funding to Johns Hopkins University from Astra Zeneca and Personal Genome Diagnostics, has received research funding to Johns Hopkins University from Bristol-Myers Squibb and Delfi Diagnostics in the past 5 years, is an advisory board member for Astra Zeneca and Neogenomics (compensated), and receives honoraria from Foundation Medicine, Guardant Health and Personal Genome Diagnostics; these arrangements have been reviewed and approved by Johns Hopkins University in accordance with its conflict-of-interest policies. V.A. is an inventor on patent applications (63/276,525, 17/779,936, 16/312,152, 16/341,862, 17/047,006 and 17/598,690) submitted by Johns Hopkins University related to cancer genomic analyses, circulating tumor DNA therapeutic response monitoring and immunogenomic features of response to immunotherapy that have been licensed to one or more entities. Under the terms of these license agreements, the university and inventors are entitled to fees and royalty distributions. W.S.M.E.T. receives research funding to the Netherlands Cancer Institute from MSD, AZ and Regeneron. N.N. is an inventor on patent application 17/598,690 submitted by Johns Hopkins University related to genomic features of response to immunotherapy. C.C. is the founder of CM Cherry Consulting. J.R.W. is the founder of Resphera Biosciences. K.N.S. has filed for patent protection on the MANAFEST technology (16/341,862), has received travel support/honoraria from Illumina, receives research funding from Bristol-Myers Squibb, Abbvie and Astra Zeneca, and owns founder’s equity in Clasp Therapeutics. V.E.V. is a founder of Delfi Diagnostics, serves on the board of directors and as a consultant for this organization, and owns Delfi Diagnostics stock, which is subject to certain restrictions under university policy. Additionally, Johns Hopkins University owns equity in Delfi Diagnostics. V.E.V. divested his equity in Personal Genome Diagnostics to LabCorp in February 2022. V.E.V. is an inventor on patent applications submitted by Johns Hopkins University related to cancer genomic analyses and cell-free DNA for cancer detection that have been licensed to one or more entities, including Delfi Diagnostics, LabCorp, Qiagen, Sysmex, Agios, Genzyme, Esoterix, Ventana and ManaT Bio. Under the terms of these license agreements, the university and inventors are entitled to fees and royalty distributions. V.E.V. is an advisor to Viron Therapeutics and Epitope; these arrangements have been reviewed and approved by the Johns Hopkins University in accordance with its conflict-of-interest policies. The other authors declare no competing interests.
Figures


References
-
- Demaria, S., Golden, E. B. & Formenti, S. C. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol.1, 1325–1332 (2015). - PubMed
-
- Kroemer, G., Galassi, C., Zitvogel, L. & Galluzzi, L. Immunogenic cell stress and death. Nat. Immunol.23, 487–500 (2022). - PubMed
-
- McLaughlin, M. et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat. Rev. Cancer20, 203–217 (2020). - PubMed
-
- Rodriguez-Ruiz, M. E. et al. Abscopal effects of radiotherapy are enhanced by combined immunostimulatory mAbs and are dependent on CD8 T cells and crosspriming. Cancer Res.76, 5994–6005 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA121113/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- P30 CA006973/CA/NCI NIH HHS/United States
- CA006973/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- R01 CA121113/CA/NCI NIH HHS/United States
- UG1 CA233259/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

