Paediatric cranial ultrasound: abnormalities of the brain in term neonates and young infants
- PMID: 40696237
- PMCID: PMC12283534
- DOI: 10.1186/s13244-025-02031-4
Paediatric cranial ultrasound: abnormalities of the brain in term neonates and young infants
Abstract
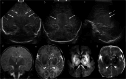
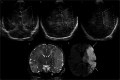
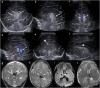
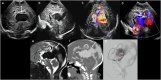
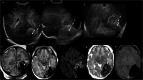
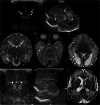
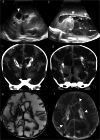
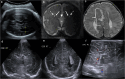
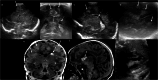
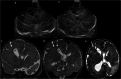
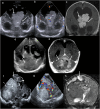
Cranial ultrasound is a critical screening tool in the detection of cerebral abnormalities in term neonates and infants, and is complementary to other imaging modalities. This pictorial review illustrates the diverse central nervous system pathologies which can affect the term neonatal and infantile brain, including vascular abnormalities (hypoxic ischaemic injury, perinatal arterial ischaemic stroke, cerebral sinovenous thrombosis, vein of Galen aneurysmal malformations, subpial haemorrhage, and dural sinus malformations); infections (congenital (cytomegalovirus and toxoplasmosis) and bacterial meningoencephalitis); genetic disorders and malformations (callosal agenesis, tuberous sclerosis, developmental megalencephaly, lissencephaly-pachygyria, and grey matter heterotopia); tumours (choroid plexus papilloma, atypical teratoid/rhabdoid tumour, and desmoplastic infantile glioma) and trauma (birth-related, inflicted injury). Each condition is explored with a focus on its sonographic characteristics-some have rarely, if ever, been described on ultrasound. CRITICAL RELEVANCE STATEMENT: Through this case review, we illustrate various pathologies affecting the term neonatal and infantile brain, including vascular lesions, infection, genetic disorders/malformations, tumours and trauma: some of these pathologies have rarely, if ever, been described on CUS. KEY POINTS: Cranial ultrasound (CUS) is a critical screening tool for the term brain. Many term neonatal and infantile pathologies can be detected on CUS. Some of the pathologies illustrated in this paper have rarely been described on US.
Keywords: Brain; Head; Infant; Pathology; Ultrasonography.
© 2025. Crown.
Conflict of interest statement
Declarations. Guarantor: Dr Rahul Lakshmanan, Medical Imaging Department, Perth Children’s Hospital, 15 Hospital Avenue, Nedlands, WA 6009, Australia. Telephone: +61 8 6456 2222. Facsimile: +61 6 6456 0072. E-mail: rahul.lakshmanan@health.wa.gov.au. Ethics approval and consent to participate: The institutional review board at the Child and Adolescent Health Service, Western Australia, approved this study and waived the need for formal ethics approval. Consent for publication: The institutional review board at the Child and Adolescent Health Service, Western Australia waived the need for individual informed consent for this project. Competing interests: The authors have no competing interests to declare.
Figures

References
-
- Ghei SK, Zan E, Nathan JE et al (2014) MR imaging of hypoxic-ischemic injury in term neonates: pearls and pitfalls. Radiographics 34:1047–1061 - PubMed
-
- Groenendaal F, de Vries LS (2017) Fifty years of brain imaging in neonatal encephalopathy following perinatal asphyxia. Pediatr Res 81:150–155 - PubMed
LinkOut - more resources
Full Text Sources

