The clinical outcomes of adjuvant gemcitabine plus S-1 in resected pancreatic cancer: a single-center retrospective study
- PMID: 40696299
- PMCID: PMC12284996
- DOI: 10.1186/s12885-025-14617-8
The clinical outcomes of adjuvant gemcitabine plus S-1 in resected pancreatic cancer: a single-center retrospective study
Abstract
Background: Adjuvant chemotherapy is the standard form of care for resected pancreatic cancer (PC) patients. Its treatment regimens include monotherapy with gemcitabine or S-1 and combination therapy with gemcitabine plus capecitabine or modified FOLFIRINOX (fluorouracil, oxaliplatin, irinotecan, leucovorin). Whether the efficacy of the adjuvant gemcitabine plus S-1 (GS) combination is realized remains uncertain.
Methods: This single-institute, retrospective, real-world study included 122 patients with resected PC from the period January 2014 to July 2021. Amongst them, 73 patients received adjuvant chemotherapy, with 21 and 35 patients receiving gemcitabine monotherapy and GS combination adjuvant chemotherapy, respectively. The clinical characteristics, outcomes and toxicities of chemotherapy were compared between these two groups.
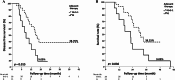
Results: The disease-free survival (DFS) and overall survival (OS) for the patients who had received GS combination were 15.8 months and 31.2 months, respectively. Compared with gemcitabine monotherapy, there was a trend towards favorable DFS (10.7 months in gemcitabine monotherapy, p = 0.083), but no OS benefits (24 months, p = 0.517) with GS combination. However, for patients in an advanced disease condition (Stages II and III), the GS combination offered statistically significant longer DFS (14.9 vs. 8.8 months; p = 0.015) and OS (31.2 vs. 21.6 months; p = 0.036), when compared with gemcitabine monotherapy. The adverse effects were comparable between the two groups.
Conclusions: In our real-world study, use of the GS combination could be another option for resected PC patients, particularly for those who are in a more advanced (Stage II and III) disease condition.
Keywords: Adjuvant chemotherapy; Disease-free survival; Gemcitabine plus S-1; Overall survival; Pancreatic cancer.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was conducted in accordance with the principles of the Declaration of Helsinki, and the institutional review board of Taichung Veterans General Hospital approved the study and waived the requirement for informed consent due to its retrospective design (No. CE22475A). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics. CA Cancer J Clin. 2022;72(1):7–33. - PubMed
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71(3):209–49. - PubMed
-
- Tanaka M, Mihaljevic AL, Probst P, Heckler M, Klaiber U, Heger U, et al. Meta-analysis of recurrence pattern after resection for pancreatic cancer. Br J Surg. 2019;106(12):1590–601. - PubMed
-
- Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350(12):1200–10. - PubMed
LinkOut - more resources
Full Text Sources

