Decoding the metabolic dialogue in the tumor microenvironment: from immune suppression to precision cancer therapies
- PMID: 40696413
- PMCID: PMC12282028
- DOI: 10.1186/s40164-025-00689-6
Decoding the metabolic dialogue in the tumor microenvironment: from immune suppression to precision cancer therapies
Abstract
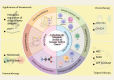
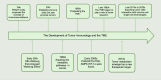
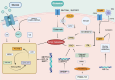
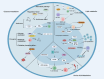
The tumor microenvironment (TME) represents a metabolic battleground where immune cells and cancer cells vie for essential nutrients, ultimately influencing antitumor immunity and treatment outcomes. Recent advancements have shed light on how the metabolic reprogramming of immune cells, including macrophages, T cells, and DCs, determines their functional polarization, survival, and interactions within the TME. Factors such as hypoxia, acidosis, and nutrient deprivation drive immune cells toward immunosuppressive phenotypes, while metabolic interactions between tumors and stromal cells further entrench therapeutic resistance. This review synthesizes new insights into the metabolic checkpoints that regulate immune cell behavior, focusing on processes like glycolysis, oxidative phosphorylation (OXPHOS), lipid oxidation, and amino acid dependencies. We emphasize how metabolic enzymes (e.g., IDO1, ACLY, CPT1A) and metabolites (e.g., lactate, kynurenine) facilitate immune evasion, and we propose strategies to reverse these pathways. Innovations such as single-cell metabolomics, spatial profiling, and AI-driven drug discovery are transforming our understanding of metabolic heterogeneity and its clinical implications. Furthermore, we discuss cutting-edge therapeutic approaches-from dual-targeting metabolic inhibitors to biomaterial-based delivery systems-that aim to reprogram immune cell metabolism and enhance the effectiveness of immunotherapy. Despite the promise in preclinical studies, challenges persist in translating these findings to clinical applications, including biomarker validation, metabolic plasticity, and interpatient variability. By connecting mechanistic discoveries with translational applications, this review highlights the potential of immunometabolic targeting to overcome resistance and redefine precision oncology.
Keywords: Immune cells metabolism; Immunotherapy resistance; Metabolic reprogramming; Therapeutic targeting; Tumor microenvironment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: All authors have agreed on the contents of the manuscript. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
An update on cancer stem cell survival pathways involved in chemoresistance in triple-negative breast cancer.Future Oncol. 2025 Mar;21(6):715-735. doi: 10.1080/14796694.2025.2461443. Epub 2025 Feb 12. Future Oncol. 2025. PMID: 39936282 Review.
-
Tumor microenvironment and immunotherapy: from bench to bedside.Med Oncol. 2025 Jun 8;42(7):244. doi: 10.1007/s12032-025-02818-x. Med Oncol. 2025. PMID: 40483667 Review.
-
Role of Tumor Microenvironment in Prostate Cancer Immunometabolism.Biomolecules. 2025 Jun 6;15(6):826. doi: 10.3390/biom15060826. Biomolecules. 2025. PMID: 40563466 Free PMC article. Review.
-
Intercellular communication between extracellular vesicles from conditioned macrophages and breast cancer cells drives endocrine therapy resistance.Front Cell Dev Biol. 2025 Jun 3;13:1548724. doi: 10.3389/fcell.2025.1548724. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40567500 Free PMC article.
-
Systematic screening of metabolic pathways to identify two breast cancer subtypes with divergent immune characteristics.Sci Rep. 2025 Jul 1;15(1):20996. doi: 10.1038/s41598-025-05179-7. Sci Rep. 2025. PMID: 40594370 Free PMC article.
References
-
- Martínez-Reyes I, Chandel NS. Cancer metabolism: looking forward. Nat Rev Cancer. 2021;21:669–80. - PubMed
Publication types
Grants and funding
- 82303610, 82471321/National Natural Science Foundation of China
- CSTB2023NSCQBHX0002/Chongqing Postdoctoral Science Foundation
- CSTB2023NSCQ-MSX0107/Natural Science Foundation Project of Chongqing, Chongqing Science and Technology Commission
- YXLJ202429/Chongqing Medical Leading Talents Program
- 20241708/Kuanren Talents Program of the Second Affiliated Hospital of Chongqing Medical University
LinkOut - more resources
Full Text Sources
Research Materials

