The influence of asthmatic inflammation and house dust mite (HDM) exposure on abundance, immune-modulatory potential, and differentiation capacity of the lung-resident mesenchymal stem cells (lrMSCs)
- PMID: 40696474
- PMCID: PMC12281997
- DOI: 10.1186/s13287-025-04520-1
The influence of asthmatic inflammation and house dust mite (HDM) exposure on abundance, immune-modulatory potential, and differentiation capacity of the lung-resident mesenchymal stem cells (lrMSCs)
Abstract
Background: Tissue-resident mesenchymal stem cells, also known as mesenchymal stromal cells (MSCs), play a crucial role in maintaining tissue homeostasis and repair. However, their function in chronic inflammatory diseases, such as asthma, remains elusive.
Aim: Here, we aimed to assess the influence of house dust mite (HDM)-induced asthmatic inflammation on the numbers and function of lung resident (lr)MSCs.
Methods: Experimental asthma was induced in female C57BL6/cmdb mice via intranasal HDM administration. LrMSCs were isolated, expanded, and characterized by flow cytometry and differentiation assays. Human adipose tissue-derived (hAD)MSCs were isolated and stimulated with HDM, LPS, or cytokines. Co-culture experiments with peripheral blood mononuclear cells (PBMCs) assessed immunomodulatory potential. Gene expression, cytokine levels, and T-cell proliferation were analyzed.
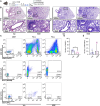
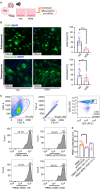
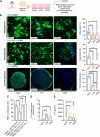
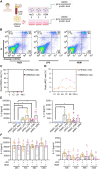
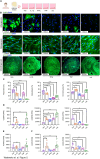
Results: Here, we showed that asthmatic lung inflammation significantly reduces the number of lrMSCs. More importantly, remaining lrMSCs showed impaired differentiation potential and lacked immunomodulatory functions. Furthermore, we found that exposure of hAD-MSCs to HDM and LPS similarly led to marked inhibition of differentiation potential and suppression of immunosuppressive activities. Notably, this inhibitory effect persisted despite the presence of pro-inflammatory cytokines released by PBMCs in response to LPS and HDM. Furthermore, we showed that inflammatory signaling alone, in the absence of direct LPS and HDM exposure, significantly reduces growth factor-induced adipogenesis and osteogenesis.
Conclusions: Taken together, our findings indicate that asthmatic inflammation not only reduces the number of lrMSCs but also impairs their function, potentially exacerbating disease progression by limiting their immunoregulatory role.
Keywords: Asthmatic lung inflammation; Immunomodulation; Lung resident MSC; MSC; MSC differentiation; MSC phenotype; Mesenchymal stem cells.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was conducted in accordance with the Declaration of Helsinki. Ethical approval was obtained from Ethics Committee of the Medical University of Bialystok (for Buffy Coat Collection Approval No: R-I-002/634/2018 approved 28 February 2019 entitled: “Assessment of human acellular dermal matrices and cellular therapies in diabetic wound healing efficacy”, for MSC collection Approval No: APK.002.114.2021 from 25.02.2021 entitled: Utilization of Mesenchymal Stem Cells, Fibroblasts, and Acellular Scaffolds Based on Human Skin Derived from Dermal-Fat Folds in Experimental Models). All animal experiments were approved by the Local Ethical Committee in Olsztyn (Approval No: number 35/2019 from 26 April 2019, entitled: Application of Human Adipose-Derived Mesenchymal Stem Cells (hADMSCs) in the Control of House Dust Mite (HDM)-Induced Allergic Lung Inflammation). The work has been reported in line with the ARRIVE guidelines 2.0. Consent for publication: All authors have read and approved the final version of this manuscript. The authors confirm that the manuscript represents original work and has not been published or submitted elsewhere, in whole or in part. Competing interests: MT reports National Science Centre (grant no. 2020/37/N/NZ5/04144); MM reports earlier personal payments from Astra Zeneca, GSK, Sanofi, Berlin-Chemie/Menarini, Chiesi, Lek-AM, Takeda, Teva, Novartis, CSL Behring, Celon, and support for attending meetings from Chiesi, Astra Zeneca, GSK, Berlin-Chemie/Menarini. AE reports National Science Centre (grant no. 2020/37/N/NZ5/04144), National Centre for Research and Development (POLTUR3/MT-REMOD/2/2019). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All authors read and accepted the manuscript.
Figures
Similar articles
-
Combustion products of burn pit constituents induce more changes in asthmatic than non-asthmatic murine lungs.Part Fibre Toxicol. 2025 Jul 29;22(1):21. doi: 10.1186/s12989-025-00625-w. Part Fibre Toxicol. 2025. PMID: 40734182 Free PMC article.
-
HGF-DPSCs ameliorate asthma by regulating CCR1+ Th2 cells responses in mice pulmonary mucosa.Cytotherapy. 2025 Jun;27(6):709-722. doi: 10.1016/j.jcyt.2025.02.005. Epub 2025 Feb 24. Cytotherapy. 2025. PMID: 40072405
-
Human Infrapatellar Fat Pad Mesenchymal Stem Cell-derived Extracellular Vesicles Purified by Anion Exchange Chromatography Suppress Osteoarthritis Progression in a Mouse Model.Clin Orthop Relat Res. 2024 Jul 1;482(7):1246-1262. doi: 10.1097/CORR.0000000000003067. Epub 2024 Apr 19. Clin Orthop Relat Res. 2024. PMID: 38662932 Free PMC article.
-
Natural compounds and mesenchymal stem cells: implications for inflammatory-impaired tissue regeneration.Stem Cell Res Ther. 2024 Feb 7;15(1):34. doi: 10.1186/s13287-024-03641-3. Stem Cell Res Ther. 2024. PMID: 38321524 Free PMC article. Review.
-
Systematic review and meta-analysis of mesenchymal stromal/stem cells as strategical means for the treatment of COVID-19.Ther Adv Respir Dis. 2023 Jan-Dec;17:17534666231158276. doi: 10.1177/17534666231158276. Ther Adv Respir Dis. 2023. PMID: 37128999 Free PMC article.
References
-
- Boonpiyathad T, Sözener ZC, Satitsuksanoa P, et al. Immunologic mechanisms in asthma. Semin Immunol. 2019;46: 101333. - PubMed
-
- Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16(1):45–56. - PubMed
-
- Hammad H, Lambrecht BN. The basic immunology of asthma. Cell. 2021;184(6):1469–85. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

