Strongyloides stercoralis prevalence and diagnostic efficacy of an IgG4 rapid test in an eosinophilic population in Khuzestan Province, southwestern Iran
- PMID: 40696476
- PMCID: PMC12281991
- DOI: 10.1186/s13071-025-06910-z
Strongyloides stercoralis prevalence and diagnostic efficacy of an IgG4 rapid test in an eosinophilic population in Khuzestan Province, southwestern Iran
Abstract
Background: Strongyloides stercoralis is a pathogenic nematode affecting the human intestine. Chronic strongyloidiasis often remains asymptomatic, posing diagnostic challenges due to the low sensitivity of conventional methods. Using traditional methods, this study investigated the prevalence of strongyloidiasis in Khuzestan Province, southwestern Iran. We also studied the effectiveness of a Strongyloides immunoglobulin G4 (IgG4) rapid diagnostic test (RDT) for timely infection detection before and after treatment.
Methods: This cross-sectional study, conducted during 2022-2024, evaluated 520 participants with eosinophilia (> 5%) for S. stercoralis infection. Coprological methods used were direct smear stool microscopy and agar plate culture. Serological methods were enzyme-linked immunosorbent assay (ELISA) (NovaTec® kit) and a prototype IgG4 RDT using a recombinant antigen (NIE) . Traditional coprology and composite references were used to assess the diagnostic power. Among copro-positive patients, 30 cases were followed up at least 3 months after treatment using the same methods.
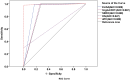
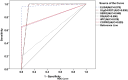
Results: Of the 373 participants who submitted stool samples, coprological methods identified 95 positive cases, with culture proving to be more sensitive than direct smear (24.9%, 93/373 versus 7.5%, 28/373). Of the 520 participants, 35.2% (183/520) and 43.7% (227/520) tested positive for S. stercoralis using ELISA and IgG4 RDT, respectively. Spearman's rank correlation between the IgG4 RDT and ELISA was significant (ρ = 0.772; P < 0.001). Despite minor discrepancies, the IgG4 RDT showed substantial agreement with the ELISA (κ = 0.776). Increased eosinophil counts were strongly associated with Strongyloides infection with a mean of 20.48% in copro-positives versus 15.22 in copro-negatives and area under the curve (AUC) of 0.741 and 0.701 for coprology and the combination of coprology and serology methods (CRS), respectively. In the 30 follow-up patients, a significant reduction in eosinophil counts (P < 0.001) was observed. Five cases (17%) remained larva-positive, and serological tests significantly increased readings/scores. Three copro-negative patients showed strong positive results on ELISA and IgG4 RDT.
Conclusions: On the basis of the obtained results, the prevalence of S. stercoralis infection among the eosinophilic population was high. This study showed that the IgG4 RDT is a reliable and efficient diagnostic tool for S. stercoralis infection. The rapid test results demonstrated significant agreement with the ELISA and effectively detected infection in eosinophilic patients, making it a suitable diagnostic test for screening, particularly in resource-limited settings.
Keywords: Strongyloides IgG4 rapid test (IgG4 RDT); Coprological methods; ELISA; Eosinophilia; Khuzestan Province, Iran; Prevalence; Strongyloidiasis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The protocol of this study was approved by the Ethics Committee of the Ahvaz Jundishapur University of Medical Sciences, approval no. IR.AJUMS.MEDICINE.REC.1402.087. Participants or their parents (children) were informed about the study’s objectives and provided written consent. Copro-positive cases were referred to an infectious disease specialist for treatment. Consent for publication: Not applicable. Competing interests: R.N. and N.S.A. developed the prototype IgG4 rapid tests, which were shipped from Malaysia to Iran. They had no part in the actual testing of samples, so the potential conflict of interest is minimal. Other authors have no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
- CMRC-0249/Cellular and Molecular Research Center, Medical Basic Sciences Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
- CMRC-0249/Cellular and Molecular Research Center, Medical Basic Sciences Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
- CMRC-0249/Cellular and Molecular Research Center, Medical Basic Sciences Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
- CMRC-0249/Cellular and Molecular Research Center, Medical Basic Sciences Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
- CMRC-0249/Cellular and Molecular Research Center, Medical Basic Sciences Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
- CMRC-0249/Cellular and Molecular Research Center, Medical Basic Sciences Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
- CMRC-0249/Cellular and Molecular Research Center, Medical Basic Sciences Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
LinkOut - more resources
Full Text Sources
Medical

