Prolactin Secretion During Postnatal Development in Artificial Rearing Rats (Rattus norvegicus)
- PMID: 40696887
- PMCID: PMC12284461
- DOI: 10.1002/dneu.22985
Prolactin Secretion During Postnatal Development in Artificial Rearing Rats (Rattus norvegicus)
Abstract
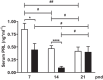
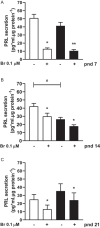
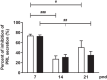
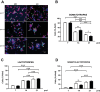
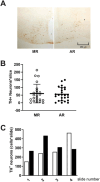
During the lactation period, rat pups are fed by their mother and are with their siblings. In the pituitary, lactotrope and somatolactotrope cells increase in number during this period and are associated with milk-borne factors. In the artificial rearing (AR) paradigm, pups are deprived of mother- and sibling-pup interactions and are fed artificial milk. AR rats present more hypothalamic neurons associated with less apoptosis during postnatal development. Moreover, AR infantile rats show growth hormone (GH), insulin-like growth factor-1 (IGF-1), and ghrelin alterations resulting from suckling behavior and the absence of meal transition at the second week of life. In the present study, the variation in prolactin (PRL) throughout the lactation period was analyzed in AR pups and compared with that in mother-reared (MR) pups. At postnatal Day 7 (pnd7), AR pups have less serum PRL than MR pups do, and a further decrease was observed at pnd14. However, only at pnd14, AR pituitary cells secrete less PRL, which was correlated with a smaller number of somatolactotrope cells unlike lactotrope cells. Analysis of the hypothalamic dopamine and DOPAC concentrations in both groups revealed no differences at 7, 14, and 21pnd. Nevertheless, the pituitary showed higher concentrations in AR pups than in MR pups at pnd14. However, the number of dopaminergic neurons in the arcuate nucleus was similar in both groups, but they were less spread in the AR pup hypothalamus. Our results revealed the importance of mothers' and siblings' interactions and mothers' milk in the maturation of the PRL hypothalamic‒pituitary axis during the lactating period.
Keywords: artificial rearing; dopamine/DOPAC; infantile period; pituitary cells; prolactin.
© 2025 The Author(s). Developmental Neurobiology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Physiological changes of growth hormone during lactation in pup rats artificially reared.PLoS One. 2019 Aug 13;14(8):e0220853. doi: 10.1371/journal.pone.0220853. eCollection 2019. PLoS One. 2019. PMID: 31408482 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection.Cochrane Database Syst Rev. 2007 Jan 24;(1):CD003510. doi: 10.1002/14651858.CD003510.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2011 Jul 06;(7):CD003510. doi: 10.1002/14651858.CD003510.pub3. PMID: 17253490 Updated.
-
Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection.Cochrane Database Syst Rev. 2011 Jul 6;(7):CD003510. doi: 10.1002/14651858.CD003510.pub3. Cochrane Database Syst Rev. 2011. PMID: 21735394
-
Role of tactile stimulation during the preweaning period on the development of the peripheral sensory sural (SU) nerve in adult artificially reared female rat.Dev Psychobiol. 2024 Jul;66(5):e22486. doi: 10.1002/dev.22486. Dev Psychobiol. 2024. PMID: 38739111
References
-
- Bero, L. A. , and Kuhn C. M.. 1987. “Differential Ontogeny of Opioid, Dopaminergic and Serotonergic Regulation of Prolactin Secretion.” Journal of Pharmacology and Experimental Therapeutics 240: 825–830. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

