Complex Interplay of Metabolic Substrates, Points of Entry into the Mitochondrial Electron Chain, and ROS Generation: A critical analysis of "Active control of mitochondrial network morphology by metabolism-driven redox state" by Singh et al. and studies replacing ETC components with yeast counterparts
- PMID: 40697051
- PMCID: PMC12376014
- DOI: 10.1002/bies.70045
Complex Interplay of Metabolic Substrates, Points of Entry into the Mitochondrial Electron Chain, and ROS Generation: A critical analysis of "Active control of mitochondrial network morphology by metabolism-driven redox state" by Singh et al. and studies replacing ETC components with yeast counterparts
Abstract
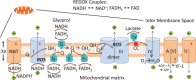
Recently, a fascinating, well-executed, molecular study regarding the direct influence of mitochondrial reactive oxygen species (ROS) formation by the electron transport chain (ETC) on mitochondrial morphology in baker's yeast appeared in PNAS. The findings highlight some very interesting connections between the choice of metabolic substrates, points of entry into the ETC, ROS formation, efficiency of ATP generation, and mitochondrial structures. These reflect both ancient eukaryotic constraints and later specific adaptations of Saccharomyces cerevisiae. However, by not addressing these adaptations, the important wider implications of the article's findings run the risk of being overlooked. There are illuminating connections to the FADH2/NADH ratio concept and new studies replacing ETC components with yeast counterparts in diverse metazoan cells, which will also be discussed.
© 2025 The Author(s). BioEssays published by Wiley‐VCH GmbH.
Conflict of interest statement
The author declares no conflict of interest.
Figures
Similar articles
-
Ischemic damage to every segment of the oxidative phosphorylation cascade elevates ETC driving force and ROS production in cardiac mitochondria.Am J Physiol Heart Circ Physiol. 2022 Sep 1;323(3):H499-H512. doi: 10.1152/ajpheart.00129.2022. Epub 2022 Jul 22. Am J Physiol Heart Circ Physiol. 2022. PMID: 35867709 Free PMC article.
-
Isa2p is essential in Saccharomyces cerevisiae for mitochondrial function and stress resistance by allowing Rip1p subunit assembly into cytochrome bc1 complex.J Biosci. 2025;50:58. J Biosci. 2025. PMID: 40642800
-
A novel inhibitor of the mitochondrial respiratory complex I with uncoupling properties exerts potent antitumor activity.Cell Death Dis. 2024 May 2;15(5):311. doi: 10.1038/s41419-024-06668-9. Cell Death Dis. 2024. PMID: 38697987 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Regulation of mitochondrial oxidative phosphorylation through tight control of cytochrome c oxidase in health and disease - Implications for ischemia/reperfusion injury, inflammatory diseases, diabetes, and cancer.Redox Biol. 2024 Dec;78:103426. doi: 10.1016/j.redox.2024.103426. Epub 2024 Nov 10. Redox Biol. 2024. PMID: 39566165 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

