Pathological complete response in advanced intrahepatic cholangiocarcinoma was achieved through tri-modal therapy: A case report and review of literature
- PMID: 40697239
- PMCID: PMC12278232
- DOI: 10.4251/wjgo.v17.i7.108650
Pathological complete response in advanced intrahepatic cholangiocarcinoma was achieved through tri-modal therapy: A case report and review of literature
Abstract
Background: Intrahepatic cholangiocarcinoma (ICC) is an aggressive malignancy with limited treatment options and a poor prognosis, particularly in unresectable or metastatic cases. Tri-modal strategies combining systemic chemotherapy, targeted therapies, and immune checkpoint inhibitors have demonstrated synergistic effects in converting unresectable ICC to resectable status and improving patient survival.
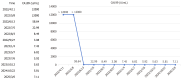
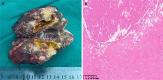
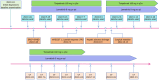
Case summary: A 39-year-old male presented with unresectable stage IIIB ICC (cT3N1M0), abdominal pain, and elevated carbohydrate antigen (CA) 19-9 levels. He received tri-modal therapy consisting of gemcitabine-oxaliplatin hepatic arterial infusion chemotherapy (GEMOX-HAIC), lenvatinib (8 mg daily), and toripalimab (160 mg every three weeks). After five cycles, significant tumor shrinkage and normalization of CA19-9 levels enabled a left hepatectomy. Complications, including biliary stenosis and liver abscesses, were managed with biliary stenting and percutaneous drainage, which allowed for the continuation of chemotherapy. Postoperative pathological examination confirmed a pathological complete response. At the last follow-up, the patient had maintained 29 months of disease-free survival post-resection and was continuing postoperative therapy.
Conclusion: This case highlights the potential of a tri-modal therapy combining GEMOX-HAIC, lenvatinib, and toripalimab to convert unresectable ICC to a resectable status, thereby potentially improving patient survival by surgical resection. Further clinical trials investigating this regimen are warranted.
Keywords: Case report; Hepatic arterial infusion chemotherapy; Intrahepatic cholangiocarcinoma; Lenvatinib; Surgery; Toripalimab.
©The Author(s) 2025. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures




References
-
- Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. - PubMed
-
- Ghiringhelli F, Lorgis V, Vincent J, Ladoire S, Guiu B. Hepatic arterial infusion of gemcitabine plus oxaliplatin as second-line treatment for locally advanced intrahepatic cholangiocarcinoma: preliminary experience. Chemotherapy. 2013;59:354–360. - PubMed
-
- Shi GM, Huang XY, Wu D, Sun HC, Liang F, Ji Y, Chen Y, Yang GH, Lu JC, Meng XL, Wang XY, Sun L, Ge NL, Huang XW, Qiu SJ, Yang XR, Gao Q, He YF, Xu Y, Sun J, Ren ZG, Fan J, Zhou J. Toripalimab combined with lenvatinib and GEMOX is a promising regimen as first-line treatment for advanced intrahepatic cholangiocarcinoma: a single-center, single-arm, phase 2 study. Signal Transduct Target Ther. 2023;8:106. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

