Neutrophil/monocyte-targeted dual-ligands modified liposomes delivering puerarin for ischemia stroke treatment
- PMID: 40697323
- PMCID: PMC12281153
- DOI: 10.1016/j.mtbio.2025.102077
Neutrophil/monocyte-targeted dual-ligands modified liposomes delivering puerarin for ischemia stroke treatment
Abstract
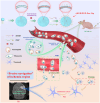
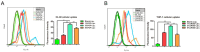
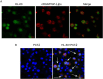
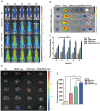
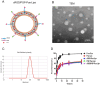
Extremely low actual biological effect of insoluble small molecule drugs in ischemia region is a pain point and aporia in Ischemia Stroke (IS) therapy, Although there are studies on single, double-ligands modified liposomes or biomimetic exogenous carriers for directly targeting IS so far, but they often have off-target effects due to they were swallowed, degraded directly (instability) and sabotaged without the help of endogenous cell in the systemic circulation. DSPE-PEG3400-PGP (PGP) and DSPE-PEG3400-cRGD (cRGD) were synthesized via michael addition reaction of maleimide (-Mal) with sulfhydryl (-SH), succinimidyl ester (-NHS) with active primary amine group (-NH2) respectively. The cRGD and PGP were modified on liposomes by thin film hydration method. Optimal modified ratio of cRGD and PGP were achieved by cellular uptake of HL-60 cells and THP-1 cells in vitro. The precise targeting effects of cRGD/PGP-Lips were examined in a nude MCAO model by an in a vivo imaging system. Puerarin (Pue) was cleverly encapsulated using a calcium acetate gradient to construct cRGD/PGP-Pue-Lips, and its therapeutic efficiency were assessed by rat MCAO model of IS. Optimal modification ratio for both cRGD and PGP were 3 %. The cRGD/PGP-Lips had significant synergetic targeting efficiency in vitro and in vivo, and the encapsulation efficiency of Pue were greater than 80 % through calcium acetate gradient. The cRGD/PGP-Pue-Lips could effectively penetrate BBB and enhance Pue retention on the brain ischemia region in vivo, resulting in a nearly two-fold reduction significantly in cerebral infarction area and edema in rats. In addition, cRGD/PGP-Pue-Lips didn't cause systemic toxicity in major organ tissues. Precise dual-ligands modified nanocarrier targeting endogenous cells is highly competitive as a novel anti-stroke and perspective for treatment of IS.
Keywords: Ischemia stroke; Monocytes; Neutrophils; PGP; cRGD.
© 2025 Published by Elsevier Ltd.
Conflict of interest statement
All of us authors unanimously declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
Figures




Similar articles
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
References
-
- Liu W., Lu H.W., Rao X.Y., Li X., Lu H.D., Li F.F., et al. Enhanced treatment for cerebral ischemia-reperfusion injury of puerarin loading liposomes through neutrophils-mediated targeted delivery. Nano Res. 2021;14:4634–4643. doi: 10.1007/s12274-021-3395-y. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

