Treatment outcomes of extensively drug-resistant tuberculosis in Europe: a retrospective cohort study
- PMID: 40697337
- PMCID: PMC12281379
- DOI: 10.1016/j.lanepe.2025.101380
Treatment outcomes of extensively drug-resistant tuberculosis in Europe: a retrospective cohort study
Abstract
Background: In 2021, World Health Organization revised of definition of extensive drug-resistant tuberculosis. We aimed to determine treatment outcomes of individuals affected by extensively drug-resistant tuberculosis in Europe.
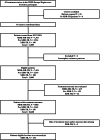
Methods: This observational, retrospective cohort study included patients diagnosed with extensively drug-resistant tuberculosis in the World Health Organization European Region from 2017 to 2023. Participating centres collected consecutive, detailed individual data for extensively drug-resistant tuberculosis patients. Data were analysed with meta- and regression methods, accounting for between-country heterogeneity.
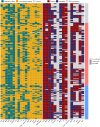
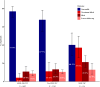
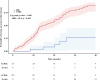
Findings: Among 11,003 patients with multidrug-resistant/rifampicin-resistant tuberculosis, 188 (1·7%) from 16 countries had extensively drug-resistant tuberculosis. Of these, 48·4% harboured strains with resistance to bedaquiline (n = 91/188), 34·0% to linezolid (n = 64/188), and 17·6% to both (n = 33/188). The individual composition of anti-tuberculosis regimens was highly variable, with 151 different drug combinations. Among the 156/188 (83·0%) patients with available treatment outcomes, the pooled percentage of successful outcomes was 40·2% (95% confidence interval [95% CI] 28·4%-53·2%). In patients with unsuccessful treatment outcomes (101/156), most experienced treatment failure (n = 57/156 [pooled proportion 37·1%], 95% CI: 26·1%-49·7%) or death (n = 30/156 [pooled proportion 21·3%], 95% CI: 15·7%-28·2%). After adjustment for disease severity, each additional likely effective drug decreased the odds of unsuccessful outcomes (adjusted odds ratio: 0·65, 95% CI: 0·45-0·96) (p = 0·026), whereas being treated in an upper-middle-income country increased the odds of unsuccessful outcomes compared with being treated in a high-income country (adjusted odds ratio: 13·7, 95% CI: 3·7-50·2) (p < 0·001). Compared with other levels of drug resistance, treatment outcomes were significantly worse for extensively drug-resistant tuberculosis.
Interpretation: Only four out of ten patients affected by extensively drug-resistant tuberculosis achieved successful treatment outcomes. These findings highlight the need for adequate, individualised treatment regimens and optimised drug susceptibility testing.
Funding: None.
Keywords: ESGMYC; MDR/RR-TB; Pre-XDR-TB; TB; TBnet; XDR-TB.
© 2025 The Author(s).
Conflict of interest statement
LG is co-Principal Investigator of two MSF-sponsored Phase III randomised controlled clinical trials (endTB & endTB-Q) testing new shorter regimens for MDR/RR-TB. Both trials are mainly funded by Unitaid. LG is PI of a Phase III clinical trial (FAST-MDR) which is funded by the French National Hospital Program for Clinical Research (PHRC) and by a pro-bono donation by Viatris. CL is the clinical lead of the UNITE4TB consortium which conducts clinical tuberculosis trials in Phase IIa-c. CL received honoraria for speaking at symposia sponsored by Astra Zeneca, Gilead, GSK, Insmed, medUpdate, MedUpdateEurope, and Pfizer outside of the scope of this study. LRC received honoraria for speaking at symposia sponsored by Viatris and participated in an advisory board for Cepheid outside of the scope of this study. All other authors declare no competing interests.
Figures
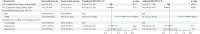
References
-
- World Health Organization . WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance. World Health Organization; Geneva, Switzerland: 2024. Licence: CC BY-NC-SA 3.0 IGO.
LinkOut - more resources
Full Text Sources

