Salivary gland transcriptomic analysis and immunophenotyping in the IL-14α transgenic mouse model of Sjögren's disease
- PMID: 40697343
- PMCID: PMC12279800
- DOI: 10.3389/fdmed.2025.1612522
Salivary gland transcriptomic analysis and immunophenotyping in the IL-14α transgenic mouse model of Sjögren's disease
Abstract
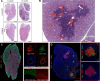
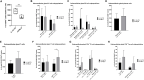
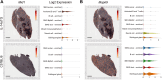
Sjögren's disease (SjD) is a systemic autoimmune disorder primarily affecting the exocrine glands and characterized by dry mouth and dry eye, the presence of anti-SSA and/or anti-SSB autoantibodies in blood serum, and chronic lymphocytic infiltration of salivary and lacrimal glands (i.e., sialadenitis and dacryoadenitis, respectively). In addition to reduced quality of life, SjD patients experience severe oral health complications and are at increased risk of developing B cell lymphoma. Because current SjD treatments primarily focus on oral and ocular symptom management, identifying initiating factors and mechanisms of disease progression may offer new therapeutic insights for SjD. The interleukin-14α transgenic (IL-14αTG) mouse model of SjD recapitulates many aspects of human SjD, including progressive sialadenitis, loss of salivary gland function, and development of B cell lymphoma. We utilized immunofluorescence, flow cytometry, bulk RNA sequencing and spatial transcriptomic analyses to identify immune cell subpopulations and differentially expressed genes (DEGs) in submandibular glands of IL-14αTG Sjögren's-like mice and age-matched C57BL/6 mouse controls. We further compared the gene ontology of DEGs in IL-14αTG mice to DEGs identified in minor salivary gland biopsies from SjD patients and healthy volunteers. Results demonstrated significantly increased sialadenitis in IL-14αTG compared to C57BL/6 mice that correlated with an increased proportion of marginal zone B cells infiltrating the submandibular gland. Whole transcriptome analyses showed substantial overlap in enriched DEG ontology between IL-14αTG mouse submandibular gland and SjD patient minor salivary gland, compared to C57BL/6 mice and healthy human volunteer controls, respectively. Lastly, we spatially resolved DEG expression and localization within IL-14αTG salivary glands, marking the first publication of a spatial transcriptomic dataset from submandibular glands in a SjD mouse model.
Keywords: RNAseq; Sjögren's disease; interleukin-14α transgenic; salivary gland; sialadenitis; spatial transcriptome.
© 2025 Woods, Jasmer, Muñoz Forti, Kearns and Weisman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjogren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. (2017) 69(1):35–45. 10.1002/art.39859 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

