A novel organoid model retaining the glioma microenvironment for personalized drug screening and therapeutic evaluation
- PMID: 40697395
- PMCID: PMC12281051
- DOI: 10.1016/j.bioactmat.2025.07.015
A novel organoid model retaining the glioma microenvironment for personalized drug screening and therapeutic evaluation
Abstract
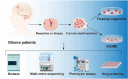
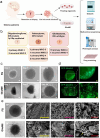
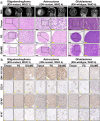
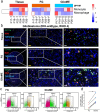
Glioma is an aggressive brain tumor with a poor prognosis. Establishing an in vitro culture model that closely replicates the cellular composition and microenvironment of the original tumor has been challenging, limiting its clinical applications. Here, we present a novel approach to generate glioma organoids with a microenvironment (GlioME) from patient-derived glioma tissue. These organoids maintain the genetic and epigenetic characteristics of the primary tumor and preserve cell-to-cell interactions within the tumor microenvironment, including resident immune cells. Bulk RNA sequencing, whole exome sequencing, and DNA methylation analysis were used to confirm the molecular similarities between the organoids and primary glioma tissues. Immunofluorescence and flow cytometry were used to assess immune cell viability, comparing GlioME with floating glioma organoids. GlioME exhibited high responsiveness to chemotherapy and targeted therapy, demonstrating its potential for therapeutic screening applications. Notably, GlioME accurately predicted patient response to the recently approved MET inhibitor, vebreltinib. Thus, this organoid model provides a reliable in vitro platform for glioma microenvironment-related research and clinical drug screening.
Keywords: Drug screening; Glioma; Matrigel; Organoid; Tumor microenvironment.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Gillet J.-P., Calcagno A.M., Varma S., Marino M., Green L.J., Vora M.I., Patel C., Orina J.N., Eliseeva T.A., Singal V., et al. Redefining the relevance of established cancer cell lines to the study of mechanisms of clinical anti-cancer drug resistance. Proc. Natl. Acad. Sci. U. S. A. 2011;108:18708–18713. doi: 10.1073/pnas.1111840108. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

