Engineered acetylated inulin nanoparticles for enhanced oral insulin delivery: sustained release, structural stability, and in vivo efficacy
- PMID: 40697464
- PMCID: PMC12282581
- DOI: 10.1039/d5ra03627e
Engineered acetylated inulin nanoparticles for enhanced oral insulin delivery: sustained release, structural stability, and in vivo efficacy
Abstract
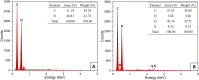
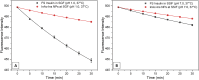
Oral insulin administration is limited by enzymatic degradation and poor gastrointestinal absorption. This study aimed to develop a biopolymer-based nanocarrier using acetylated inulin (InAc) to improve the structural stability and oral bioavailability of insulin. Inulin was produced from Salinivibrio sp. GM01 and chemically modified via acetylation. Insulin-loaded InAc (InAc-Ins) nanoparticles were prepared and characterized for morphology, size, zeta potential, and encapsulation efficiency. In vitro insulin release was evaluated under simulated gastric (SGF) and small intestinal (SSIF) conditions. In vivo efficacy was determined through oral glucose tolerance tests (OGTT) in mice. The InAc-Ins nanoparticles were spherical with mean diameter of 349 ± 38 nm and high encapsulation efficiency (92.14%). Insulin release half-life were observed in 37.1 hours in SGF and 24.3 hours in SSIF conditions. Biophysical analysis revealed enhanced structural stability of encapsulated insulin, with increased half-life and activation energy for the secondary and tertiary structure denaturation. The secondary structure denaturation half-life increased to 195 min (SGF) and 231 min (SSIF), with denaturation enthalpy of 4.03 kcal mol-1 and 1.83 kcal mol-1, respectively. Tertiary structure denaturation half-life were 765 min (SGF) and 919 min (SSIF), and denaturation enthalpy of 18.67 kcal mol-1 and 4.58 kcal mol-1, respectively. OGTT results showed that orally administered InAc-Ins nanoparticles reduced blood glucose levels more effectively than free insulin, achieving 42.8% of subcutaneous insulin efficacy. InAc nanoparticles offer effective protection and sustained release of insulin under gastrointestinal conditions, enhancing its structural integrity and hypoglycemic efficacy. This platform presents a promising strategy for non-invasive oral insulin delivery.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflicts of interest in relation to this work. There are no financial or personal relationships that could be viewed as potential competing interests.
Figures





References
-
- Antar S. A. Ashour N. A. Sharaky M. Khattab M. Ashour N. A. Zaid R. T. Roh E. J. Elkamhawy A. Al-Karmalawy A. A. Biomed. Pharmacother. 2023;168:115734. - PubMed
-
- Xiao Y. Tang Z. Wang J. Liu C. Kong N. Farokhzad O. C. Tao W. Angew. Chem., Int. Ed. 2020;59:19787–19795. - PubMed
-
- Iyer G. Dyawanapelly S. Jain R. Dandekar P. Int. J. Biol. Macromol. 2022;208:565–585. - PubMed
LinkOut - more resources
Full Text Sources

