Decoding androgen excess in polycystic ovary syndrome: Roles of insulin resistance and other key intraovarian and systemic factors
- PMID: 40697592
- PMCID: PMC12278101
- DOI: 10.4239/wjd.v16.i7.108789
Decoding androgen excess in polycystic ovary syndrome: Roles of insulin resistance and other key intraovarian and systemic factors
Abstract
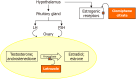
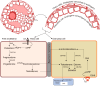
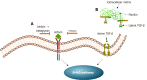
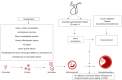
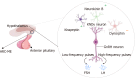
Recent studies have potentiated the essential role of androgens in normal folliculogenesis and, therefore, female fertility. Contrastingly, excess androgen levels, i.e., hyperandrogenism (HA), a hallmark characteristic of polycystic ovary syndrome, overrides the delicate balance of folliculogenesis, leading to follicular arrest and ovulatory issues. Insulin resistance (IR) has a profound effect on elevating androgen secretion and is considered one of the primary factors driving both ovarian androgen production and metabolic dysfunction in polycystic ovary syndrome. Together with IR, disruptions in key intraovarian and systemic factors, including activin, inhibin, follistatin, anti-Mullerian hormone, bone morphogenetic proteins, growth differentiation factor-9 and Kit ligand, as well as dysregulation in both the insulin and the transforming growth factor-β superfamily signaling pathway, contribute to follicular arrest, elevated androgen levels and metabolic dysfunction, exacerbating HA. Additionally, suppression of sex hormone-binding globulin, disrupted adipose-neuroendocrine signaling and altered microRNA expression heighten HA, with IR serving as the fundamental contributor. Emerging evidence implicates impaired atresia together with non-apoptotic cell death, such as ferroptosis and pyroptosis, which have also been associated with ovarian dysfunction. A comprehensive understanding of the most significant factors, particularly IR, which amplifies androgen production through hyperinsulinemia-mediated stimulation of theca cells, is essential for identifying targeted therapeutic strategies.
Keywords: Folliculogenesis; Hyperandrogenism; Insulin resistance; Obesity; Ovary; Oxidative stress; Polycystic ovary syndrome.
©The Author(s) 2025. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures

References
-
- Zirak Sharkesh E, Keshavarz SA, Nazari L, Abbasi B. The dietary inflammatory index is directly associated with polycystic ovary syndrome: A case-control study. Clin Endocrinol (Oxf) 2022;96:698–706. - PubMed
-
- Chen Y, Wang G, Chen J, Wang C, Dong X, Chang HM, Yuan S, Zhao Y, Mu L. Genetic and Epigenetic Landscape for Drug Development in Polycystic Ovary Syndrome. Endocr Rev. 2024;45:437–459. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

