Protocol for the comparison of remote versus face-to-face osteoarthritis management programs (COASTAL): A randomized implementation trial
- PMID: 40697621
- PMCID: PMC12281381
- DOI: 10.1016/j.ocarto.2025.100647
Protocol for the comparison of remote versus face-to-face osteoarthritis management programs (COASTAL): A randomized implementation trial
Abstract
Objective: To determine the comparative effectiveness of three evidence-informed approaches to delivering osteoarthritis (OA) care in real-world settings: face-to-face services, telehealth services or a mobile application (app).
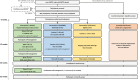
Design: COASTAL (ACTRN12624000996561) comprises two aims: the primary aim is a three-arm, non-inferiority, comparative-effectiveness, type I hybrid implementation RCT; and the secondary aim is a superiority trial to compare the three interventions separately to an untreated, non-randomized control group. We will recruit 1348 participants (primary aim:1011 interventions; secondary aim:1011 interventions, 337 controls) with knee OA referred to a NSW public Osteoarthritis Chronic Care Program (OACCP). Eligible participants who consent to OACCP participation will be randomized 1:1:1 to receive care through the face-to-face OACCP, a telehealth service, or the "OA Coach" app, over 6-months. All participants will receive a standardized needs assessment; OA education; collaboratively developed, personalized management plan with a focus on therapeutic exercise, physical activity, pain management, and weight-management, according to each arm's mode of delivery. People who have declined OACCP participation will be eligible for the control group. The primary outcome is change in average knee pain during walking at 6-months (11-point NRS). Secondary, implementation, and economic evaluation outcomes will be collected at 6 and 12-months.The protocol was co-developed with people with knee OA, clinicians delivering the services, and other stakeholders. Ethical approval granted by the Northern Sydney Local Health District (2024/ETH01461).
Conclusion: The results of this trial will provide critical evidence to help people with OA, clinicians, service planners and policymakers choose between different modes of delivering care for knee OA.
Keywords: Implementation; Knee; Management programs; Osteoarthritis; Randomized controlled trial.
© 2025 The Author(s).
Conflict of interest statement
DJH is employed by the University of Sydney and Royal North Shore Hospital. His salary support for the University of Sydney is provided by Arthritis Australia and an NHMRC Investigator Grant Leadership 2 (#1194737). In addition, DJH is the editor of the osteoarthritis section for UpToDate, co-Editor in Chief of Osteoarthritis and Cartilage and board member of Osteoarthritis Research Society International. DJH provides consulting advice on scientific advisory boards for Pfizer, Lilly, TLCBio, Novartis, Tissuegene, and Biobone. JLB is supported by an unrestricted Fellowship from Haleon Australia, which has no relationship to this project. JPE is supported by a National Health and Medical Research Council Investigator Grant (#2025504). BFD is a member of the Senior Leadership Team of the MindSpot Clinic. He has developed several remotely-delivered psychological programs for a range of conditions, however receives no payment or royalties for their use. AMB is supported, in part, by an NHMRC-funded MRFF grant (#2016567). MMD is the recipient of a University of Melbourne Dame Kate Campbell Fellowship. In addition, MMD receives research support for investigator-initiated studies, paid to her institution, from the Medical Research Future Fund, NHMRC, Eli Lilly, Victorian Orthopedic Foundation, Australian Orthopaedic Association Research Foundation, HCF Foundation, University of Melbourne, St. Vincent's Hospital Research Foundation, and Arthritis & Osteoporosis Western Australia. MMD also reports receiving payment for provision of advice on guideline development for the Royal Australian College of GPs; sitting fees as a member of the Osteoarthritis Clinical Research Group Data & Safety Monitoring Board; and reported being a Board Director of the Australian Orthopaedic Association Research Foundation. LD receives royalties from UptoDate. CB is the Director of HealthChange Associates that provides HealthChange® Methodology training and consulting services.
Figures
References
-
- Runciman W.B., Hunt T.D., Hannaford N.A., Hibbert P.D., Westbrook J.I., Coiera E.W., et al. CareTrack: assessing the appropriateness of health care delivery in Australia. Med. J. Aust. 2012;197(2):100–105. - PubMed
-
- Hunter D.J., Bowden J.L. Therapy: are you managing osteoarthritis appropriately? Nat. Rev. Rheumatol. 2017;13(12):703–704. - PubMed
-
- Meneses S.R., Goode A.P., Nelson A.E., Lin J., Jordan J.M., Allen K.D., et al. Clinical algorithms to aid osteoarthritis guideline dissemination. Osteoarthr. Cartil. 2016;24(9):1487–1499. - PubMed
-
- Hunter D.J., Schofield D., Callander E. The individual and socioeconomic impact of osteoarthritis. Nat. Rev. Rheumatol. 2014;10(7):437–441. - PubMed
-
- Brand C.A., Harrison C., Tropea J., Hinman R.S., Britt H., Bennell K. Management of osteoarthritis in general practice in Australia. Arthritis Care Res. (Hoboken) 2014;66(4):551–558. - PubMed
LinkOut - more resources
Full Text Sources