Metal-Based Regenerative Strategies for Peripheral Nerve Injuries: From Biodegradable Ion Source to Stable Conductive Implants
- PMID: 40697648
- PMCID: PMC12280558
- DOI: 10.34133/bmr.0219
Metal-Based Regenerative Strategies for Peripheral Nerve Injuries: From Biodegradable Ion Source to Stable Conductive Implants
Abstract
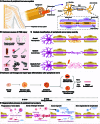
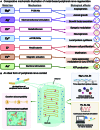
Peripheral nerve injury is a common health issue in modern aging societies, with the only treatment available being autograft transplantation. Unfortunately, autograft is often limited due to donor availability and immune rejection. Additionally, the peripheral nervous system has limited regenerative capacity, making the treatment of peripheral nerve injuries challenging. Metal-based regenerative medicine and tissue engineering strategies provide advanced solutions to the problem. Metal-based biomaterials such as conduits, filaments, alloys, hydrogels, and ceramics can deliver biofunctional metal ions and promote axonal growth and functional recovery. In parallel, metal-based electromagnetic stimulation demonstrates potential for nerve regeneration and inflammation regulation. The potential of metal-based biomaterials in promoting peripheral nerve regeneration highlights the need for further research in tissue engineering and regenerative medicine. However, rapid degradation, long-term biocompatibility, and necessary optimization regarding injury types remain to be explored. This review summarizes the reported metal-based biomaterials utilized in peripheral nerve regeneration research. The aim is to showcase advanced technologies available in the field, which may potentially become a viable alternative to autografts, offering transformative applications in the regenerative medical field.
Copyright © 2025 Hyewon Kim et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures


References
-
- Murphy RNA, Schoulepnikoff C, Chen JHC, Columb MO, Bedford J, Wong JK, Reid AJ. The incidence and management of peripheral nerve injury in England (2005–2020). J Plast Reconstr Aesthet Surg. 2023;80:75–85. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

