CRISPR/Cas9 technology in tumor research and drug development application progress and future prospects
- PMID: 40697666
- PMCID: PMC12279886
- DOI: 10.3389/fphar.2025.1552741
CRISPR/Cas9 technology in tumor research and drug development application progress and future prospects
Abstract
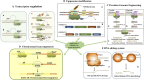
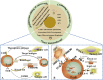
The CRISPR/Cas9 system is an acquired immune defense mechanism that has evolved in bacteria and archaea to protect against viral and plasmid attacks. It consists of regularly spaced clusters of short palindromic repeats (CRISPR) and CRISPR-associated proteins (Cas). By adapting the simplest type II CRISPR system to utilize special small guide RNA (sgRNA) and Cas9 nucleic acid endonuclease, precise cuts can be made at specific locations in double-stranded DNA, facilitating gene knockout or knock-in. Due to its efficient gene editing capabilities, CRISPR/Cas9 technology has been widely adopted across various biological and scientific research fields, demonstrating significant potential in tumor research and drug development. This article reviews the progress and future prospects of CRISPR/Cas9 technology in tumor genome editing, drug target screening and validation, and new drug development. It details the fundamental role of this technology in cancer biology research, encompassing various aspects such as gene transcription editors, epigenetic editors, precision genome engineering, and CRISPR-Cas systems targeting RNA. Additionally, the article discusses key applications of CRISPR/Cas9 in anticancer drug discovery, including drug target identification, drug target screening and validation, combinatorial genetic screening, screening of small molecules to overcome resistance to CAR-T therapies, and multimodal functional genomics integration strategies. Finally, although CRISPR/Cas9 has demonstrated great potential for efficient gene editing, precise target discovery, and promotion of personalized therapy and drug screening in oncology research, its application still faces technical bottlenecks such as off-target effects, genomic instability, and low editing efficiency in solid tumors, as well as ethical controversies in gene editing, safety assessment of delivery systems and immune responses in clinical translation, and other ethical and translational challenges.
Keywords: CRISPR/Cas9 technology; cancer therapy; drug target screening; gene editing; tumor research.
Copyright © 2025 Han, Sun, Guo, Wen, Zhao and Zhou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Arnan C., Ullrich S., Pulido-Quetglas C., Nurtdinov R., Esteban A., Blanco-Fernandez J., et al. (2022). Paired guide RNA CRISPR-Cas9 screening for protein-coding genes and lncRNAs involved in transdifferentiation of human B-cells to macrophages. BMC Genomics 23 (1), 402. 10.1186/s12864-022-08612-7 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

