Adjuvant HAIC combined with anlotinib and TQB2450 for resected high-risk hepatocellular carcinoma
- PMID: 40697792
- PMCID: PMC12277749
- DOI: 10.1016/j.xinn.2025.100910
Adjuvant HAIC combined with anlotinib and TQB2450 for resected high-risk hepatocellular carcinoma
Abstract
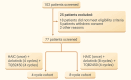
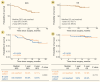
In this single-arm, phase 2 study, we explored the efficacy and safety of hepatic arterial infusion chemotherapy (HAIC) combined with anlotinib and TQB2450 (an anti-PD-L1 antibody) as a postoperative adjuvant treatment in patients at high risk of hepatocellular carcinoma (HCC) recurrence. Patients with high-risk recurrence of HCC were treated with HAIC, anlotinib (4 or 8 cycles), and TQB2450 after curative surgery. The primary endpoint was disease-free survival (DFS), and the secondary endpoints included overall survival (OS) and safety. 77 patients who met the inclusion criteria were enrolled: 38 in the 4-cycle cohort and 39 in the 8-cycle cohort. As of the data cutoff on June 24, 2024, the median follow-up period was 21.19 months (95% confidence interval [CI], 20.49-21.89). The median DFS and OS of all patients were not reached. 1-year and 2-year DFS rates were 84.4% and 65.8%, respectively, while 1-year and 2-year OS rates were 96.1% and 89.8%, respectively. No significant differences in DFS and OS were observed in patients treated with 4 or 8 cycles of anlotinib. The incidence of grade 3/4 adverse events (AEs) was 28.6%. Postoperative adjuvant HAIC combined with anlotinib and TQB2450 demonstrated encouraging clinical benefits in reducing recurrence with manageable toxicities in patients at high risk of HCC recurrence. 4-cycle anlotinib combined with HAIC and TQB2450 is recommended as an adjuvant treatment for further investigation.
Keywords: TQB245; adjuvant therapy; anlotinib; hepatic arterial infusion chemotherapy; hepatocellular carcinoma; recurrence.
© 2025 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
The efficacy and safety of tislelizumab with or without tyrosine kinase inhibitor as adjuvant therapy in hepatocellular carcinoma with high-risk of recurrence after curative resection.Front Immunol. 2025 Jun 18;16:1593153. doi: 10.3389/fimmu.2025.1593153. eCollection 2025. Front Immunol. 2025. PMID: 40607378 Free PMC article.
-
Postoperative adjuvant chemotherapy in rectal cancer operated for cure.Cochrane Database Syst Rev. 2012 Mar 14;2012(3):CD004078. doi: 10.1002/14651858.CD004078.pub2. Cochrane Database Syst Rev. 2012. PMID: 22419291 Free PMC article.
-
Conversion study of hepatocellular carcinoma using HAIC combined with lenvatinib and PD-1/L1 immunotherapy under the guidance of BCLC staging.Front Immunol. 2025 Jun 2;16:1596864. doi: 10.3389/fimmu.2025.1596864. eCollection 2025. Front Immunol. 2025. PMID: 40529364 Free PMC article.
-
Optimisation of chemotherapy and radiotherapy for untreated Hodgkin lymphoma patients with respect to second malignant neoplasms, overall and progression-free survival: individual participant data analysis.Cochrane Database Syst Rev. 2017 Sep 13;9(9):CD008814. doi: 10.1002/14651858.CD008814.pub2. Cochrane Database Syst Rev. 2017. PMID: 28901021 Free PMC article.
-
Comparison of the efficacy and safety of TACE-HAIC-MTTs-ICIs and TACE-MTTs-ICIs in the hepatocellular carcinoma: a prognostic analysis based on the dynamic changes of serum AFP.Int J Surg. 2025 Jun 20. doi: 10.1097/JS9.0000000000002818. Online ahead of print. Int J Surg. 2025. PMID: 40540455
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

