Lactobacillus rhamnosus D3189 modulates antiviral and inflammatory responses in primary nasal epithelial cells, reducing respiratory syncytial virus shedding
- PMID: 40697815
- PMCID: PMC12279880
- DOI: 10.3389/fcimb.2025.1625517
Lactobacillus rhamnosus D3189 modulates antiviral and inflammatory responses in primary nasal epithelial cells, reducing respiratory syncytial virus shedding
Abstract
Introduction: Respiratory syncytial virus (RSV) infection in the upper respiratory tract promotes disease progression and transmission, with excessive inflammation contributing to severe lower respiratory tract involvement. This study investigates the immunomodulatory effects of Lactobacillus rhamnosus D3189 on viral kinetics and innate immune responses in well-differentiated nasal epithelial cells (WD-NECs).
Methods: WD-NECs from healthy adult donors (N = 8) were cultured in vitro, treated with L. rhamnosus D3189, and then infected with RSV (strain RS4) 24 hours later. Viral replication and shedding were assessed via RT-qPCR and plaque assays. Cytotoxicity and epithelial integrity were evaluated using LDH release and transepithelial electrical resistance (TEER). Inflammatory and antiviral responses were investigated using multiplex immunoassays, AlphaLISA, and ELISA.
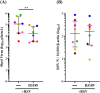
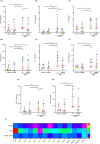
Results: RSV infection induced robust viral replication and shedding, disrupted epithelial barrier integrity, and triggered the release of pro-inflammatory cytokines and type I/III interferons. L. rhamnosus D3189 alone did not induce cytotoxicity or inflammation. While it had no effect on viral replication, TEER, LDH release, or IFN-λ1/3 levels, D3189 significantly enhanced IFN-β production, reduced viral shedding, and attenuated RSV-induced cytokine and chemokine responses.
Discussion: L. rhamnosus D3189 modulates the epithelial immune response to RSV, reducing inflammation and viral shedding without compromising epithelial integrity. These findings support its potential as a novel strategy to limit RSV-associated infection and transmission.
Keywords: antiviral; inflammation; innate immunity; lactobacilli; nasal epithelium; respiratory syncytial virus.
Copyright © 2025 Yarlagadda, Stedman, Maresco-Pennisi, Coleman, Cervin and Spann.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Lactobacillus rhamnosus dampens cytokine and chemokine secretion from primary human nasal epithelial cells infected with rhinovirus.J Appl Microbiol. 2024 Feb 1;135(2):lxae018. doi: 10.1093/jambio/lxae018. J Appl Microbiol. 2024. PMID: 38268489
-
Elimination of gut microbiota hinders the therapeutic effect of amentoflavone on respiratory syncytial virus-induced lung inflammation injury by regulating innate immunity.Phytomedicine. 2025 Sep;145:157033. doi: 10.1016/j.phymed.2025.157033. Epub 2025 Jul 8. Phytomedicine. 2025. PMID: 40701130
-
Polyanhydride nanoparticles encapsulating innate sensor agonists activate epithelial and airway cells and reduce Respiratory Syncytial Virus infection in mice.Acta Biomater. 2025 Jul 1;201:501-516. doi: 10.1016/j.actbio.2025.05.061. Epub 2025 May 24. Acta Biomater. 2025. PMID: 40419069
-
Palivizumab for immunoprophylaxis of respiratory syncytial virus (RSV) bronchiolitis in high-risk infants and young children: a systematic review and additional economic modelling of subgroup analyses.Health Technol Assess. 2011 Jan;15(5):iii-iv, 1-124. doi: 10.3310/hta15050. Health Technol Assess. 2011. PMID: 21281564 Free PMC article.
-
Host immune response to respiratory syncytial virus infection and its contribution to protection and susceptibility in adults: a systematic literature review.Expert Rev Clin Immunol. 2025 Jun;21(6):745-760. doi: 10.1080/1744666X.2025.2494658. Epub 2025 May 2. Expert Rev Clin Immunol. 2025. PMID: 40278893 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

