Plasma protein biomarkers of Plasmodium falciparum infection in pregnant women: a high-throughput proteomics study
- PMID: 40697816
- PMCID: PMC12279802
- DOI: 10.3389/fcimb.2025.1594088
Plasma protein biomarkers of Plasmodium falciparum infection in pregnant women: a high-throughput proteomics study
Abstract
Introduction: Pregnant women in sub-Saharan Africa face heightened susceptibility to Plasmodium falciparum malaria, with placental sequestration driving adverse outcomes. The infection may lead to pregnancy-associated malaria (PAM) because of the sequestration of Plasmodium falciparum-infected erythrocytes in the placental intervillous space. Although there are several tools for diagnosing malaria infection during pregnancy, including blood smear microscopic examination, rapid diagnostic tests, and PCR, there are no tools for detecting placental infection and, by extension, any dysfunction associated with PAM. Thus, PAM, specifically placental infection, can only be confirmed via postnatal placental histopathology. Therefore, there is an urgent need for specific plasma biomarkers of PAM.
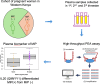
Methods: Here, we used the high throughput proximity extension assay to screen plasma from malaria-exposed pregnant women for differentially expressed proteins that may serve as candidate biomarkers of Plasmodium falciparum infection during pregnancy, with future potential to inform diagnosis of PAM or adverse malaria outcomes. Such biomarkers may also elucidate the pathophysiology of PAM.
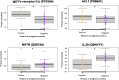
Results: Using proximity extension assay (PEA), we identified elevated IgG Fc receptor IIb (FCGR2B) and heme oxygenase-1 (HO-1) in malaria-positive pregnancies, while neurturin (NRTN) and IL-20 were downregulated.
Discussion: IL-20 emerged as a top candidate biomarker, warranting validation in large cohorts with placental histopathology.
Keywords: Plasmodium falciparum; biomarkers; malaria in pregnancy; pregnancy-associated malaria; proteomics; proximity extension assay (PEA).
Copyright © 2025 Kanoi, Waweru, Kobia, Mukala, Kirira, Mogere, Gallini, Åberg, Vatish, Gitaka and Kamali-Moghaddam.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
Systematic review and meta-analysis: rapid diagnostic tests versus placental histology, microscopy and PCR for malaria in pregnant women.Malar J. 2011 Oct 28;10:321. doi: 10.1186/1475-2875-10-321. Malar J. 2011. PMID: 22035448 Free PMC article.
-
Primaquine for reducing Plasmodium falciparum transmission.Cochrane Database Syst Rev. 2012 Sep 12;(9):CD008152. doi: 10.1002/14651858.CD008152.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2014 Jun 30;(6):CD008152. doi: 10.1002/14651858.CD008152.pub3. PMID: 22972117 Updated.
-
Use of biochemical tests of placental function for improving pregnancy outcome.Cochrane Database Syst Rev. 2015 Nov 25;2015(11):CD011202. doi: 10.1002/14651858.CD011202.pub2. Cochrane Database Syst Rev. 2015. PMID: 26602956 Free PMC article.
-
Mefloquine for preventing malaria in pregnant women.Cochrane Database Syst Rev. 2018 Mar 21;3(3):CD011444. doi: 10.1002/14651858.CD011444.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2018 Nov 14;11:CD011444. doi: 10.1002/14651858.CD011444.pub3. PMID: 29561063 Free PMC article. Updated.
-
Exploring the P. falciparum antigens associated with reduced risk of malaria in pregnancy.Front Immunol. 2025 Jul 14;16:1622435. doi: 10.3389/fimmu.2025.1622435. eCollection 2025. Front Immunol. 2025. PMID: 40726979 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

