Crossing the barrier or how regulation of ovastacin controls fertilization and translates into clinical phenotypes
- PMID: 40697826
- PMCID: PMC12281054
- DOI: 10.1016/j.isci.2025.112976
Crossing the barrier or how regulation of ovastacin controls fertilization and translates into clinical phenotypes
Abstract
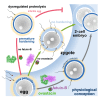
The zona pellucida, a glycoprotein matrix enveloping the mammalian egg, exerts essential functions during fertilization and early embryonic development. Its safeguard property regulates sperm entry and thus indirectly controls fertility. Limited proteolysis by the metalloproteinase ovastacin, released from the egg during fertilization, induces hardening of the zona pellucida. This precludes sperm entry and protects the embryo until implantation. However, ovastacin leakage before fertilization causes premature hardening and infertility if activity is not inhibited. This highlights the importance of ovastacin regulation by its endogenous inhibitor, fetuin-B. Accordingly, both loss and excessive ovastacin activity are linked to infertility. Here, we review recent discoveries on how ovastacin is precisely controlled to preserve zona pellucida permeability prior to fertilization and prevent penetration afterward. Based on these molecular mechanisms, we propose explanations for clinical phenotypes of recently discovered genetic mutations in ovastacin and discuss how modulation of ovastacin activity might be employed to regulate fertilization.
Keywords: Biochemistry; Cell biology; Developmental biology.
© 2025 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Improving sperm selection strategies for assisted reproduction through closing the knowledge gap in sperm maturation mechanics.Hum Reprod Open. 2025 Jul 3;2025(3):hoaf040. doi: 10.1093/hropen/hoaf040. eCollection 2025. Hum Reprod Open. 2025. PMID: 40698010 Free PMC article. Review.
-
Substrate profiling of the metalloproteinase ovastacin uncovers specific enzyme-substrate interactions and discloses fertilization-relevant substrates.FEBS J. 2024 Jan;291(1):114-131. doi: 10.1111/febs.16954. Epub 2023 Sep 21. FEBS J. 2024. PMID: 37690456
-
Intracellular activation of ovastacin mediates pre-fertilization hardening of the zona pellucida.Mol Hum Reprod. 2017 Sep 1;23(9):607-616. doi: 10.1093/molehr/gax040. Mol Hum Reprod. 2017. PMID: 28911209 Free PMC article.
-
The role of carbohydrate recognition during human sperm-egg binding.Hum Reprod. 2013 Mar;28(3):566-77. doi: 10.1093/humrep/des447. Epub 2013 Jan 12. Hum Reprod. 2013. PMID: 23315069
-
The "life code": A theory that unifies the human life cycle and the origin of human tumors.Semin Cancer Biol. 2020 Feb;60:380-397. doi: 10.1016/j.semcancer.2019.09.005. Epub 2019 Sep 12. Semin Cancer Biol. 2020. PMID: 31521747 Review.
References
-
- Matorras R., Chaudhari V.S., Roeder C., Schwarze J.E., Bühler K., Hwang K., Chang-Woo C., Iniesta S., D’Hooghe T., Mathur R. Evaluation of costs associated with fertility treatment leading to a live birth after one fresh transfer: A global perspective. Best Pract. Res. Clin. Obstet. Gynaecol. 2023;89 doi: 10.1016/j.bpobgyn.2023.102349. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

