Impact of delayed addition of PD-1/PD-L1 inhibitors to chemotherapy on outcomes in patients with extensive-stage small cell lung cancer
- PMID: 40697877
- PMCID: PMC12280550
- DOI: 10.1177/17588359251356919
Impact of delayed addition of PD-1/PD-L1 inhibitors to chemotherapy on outcomes in patients with extensive-stage small cell lung cancer
Abstract
Background: Immunotherapy combined with chemotherapy is the first-line treatment for extensive-stage small cell lung cancer (ES-SCLC). However, the effect of delayed initiation of immunotherapy on its efficacy remains unclear.
Objectives: This study aimed to investigate the impact of the delayed addition of programmed death receptor 1/programmed death ligand 1 (PD-1/PD-L1) inhibitors on treatment outcomes in patients with ES-SCLC.
Design: This retrospective cohort study used propensity score matching (PSM) to balance baseline characteristics.
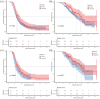
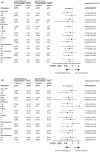
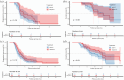
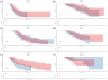
Methods: This study included 416 patients with ES-SCLC who received first-line immunotherapy between January 2020 and December 2022. Patients were categorized into two groups: delayed-immunotherapy (IO) (PD-1/PD-L1 inhibitors initiated during the 2nd or 3rd chemotherapy cycle) and early-IO (immunotherapy initiated during the first cycle). A 1:1 PSM was performed to balance baseline characteristics. The primary endpoints were overall survival (OS) and progression-free survival (PFS), which were analyzed using the Kaplan-Meier method and compared using log-rank tests.
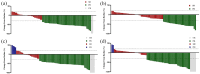
Results: Owing to the exclusion of PD-L1/PD-1 inhibitors from medical insurance, financial constraints, and poor physical condition of some patients, 72 patients were included in the delayed-IO group (second cycle: 41; third cycle: 31), while 344 were in the early-IO group. Before PSM, median OS and PFS in the delayed-IO group were 24.00 and 8.75 months, compared to 18.59 and 7.57 months in the early-IO group, with no significant differences (OS: HR 0.72, p = 0.054; PFS: HR 0.86, p = 0.281). After PSM (72 patients per group), the delayed-IO group showed significantly longer median OS (24.00 vs 18.79 months, HR 0.60, 95% CI 0.38-0.97, p = 0.037) and PFS (8.75 vs 6.49 months, HR 0.69, 95% CI 0.48-0.99, p = 0.044) compared to the early-IO group. These results suggest that, in specific patient populations, delayed immunotherapy may improve survival outcomes by optimizing patient condition or treatment response.
Conclusion: In patients with ES-SCLC, delayed administration of PD-1/PD-L1 inhibitors during the second to fourth chemotherapy cycles improves survival outcomes compared to concurrent administration during the first cycle, with a similar safety profile. These results suggest that, in specific patient populations, delayed immunotherapy may improve survival outcomes by optimizing patient condition or treatment response.
Keywords: chemotherapy; combined timing; extensive-stage small cell lung cancer (ES-SCLC); immune checkpoint inhibitor; propensity score-matched study.
Plain language summary
The impact of delayed immunotherapy on treatment outcomes in small-cell lung cancer This study looks at the best time to start immunotherapy for small-cell lung cancer, which is a very aggressive type of lung cancer often found in its advanced stage. The standard treatment now is to combine immunotherapy with chemotherapy. However, it’s unclear if when you start immunotherapy affects how well it works. The study included 416 patients divided into two groups: one group started immunotherapy during the 2nd or 3rd cycle of chemotherapy (delayed immunotherapy group), and the other group started it with the first cycle of chemotherapy (early immunotherapy group). After matching patients’ basic characteristics, the delayed group had longer survival (median overall survival of 24 months) compared to the early group (median overall survival of 18.8 months). The delayed group also had longer progression-free survival (median 8.75 months) compared to the early group (median 6.49 months). This suggests that starting immunotherapy later, during the 2nd to 4th chemotherapy cycles, may be more effective than starting it with the first cycle. Importantly, delayed immunotherapy did not increase safety concerns. This finding offers new insights for treating small-cell lung cancer, especially for patients who might face financial or health-related reasons for not starting immunotherapy immediately.
© The Author(s), 2025.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures

References
-
- Megyesfalvi Z, Gay CM, Popper H, et al. Clinical insights into small cell lung cancer: tumor heterogeneity, diagnosis, therapy, and future directions. CA Cancer J Clin 2023; 73(6): 620–652. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

