Factors associated with and kinetics of anti-IFN-α autoantibodies in RAG1/2 deficiency
- PMID: 40697949
- PMCID: PMC12281840
- DOI: 10.1016/j.jacig.2025.100521
Factors associated with and kinetics of anti-IFN-α autoantibodies in RAG1/2 deficiency
Abstract
Background: Autoantibodies against IFN-α (anti-IFN-α) have been reported in recombinase activating gene (RAG) deficiency, attributed to impaired central and peripheral T-cell/B-cell tolerance. However, the clinical features, especially viral infections, associated with these autoantibodies at baseline, their kinetics over time, and their response to hematopoietic cell transplantation are not well defined.
Objective: We described the clinical and immunologic findings linked to anti-IFN-α IgG in RAG deficiency and tracked its kinetics longitudinally, including in those who underwent hematopoietic cell transplantation.
Methods: We measured anti-IFN-α IgG by enzyme-linked immunosorbent assay in 80 RAG-deficient patients with curated clinical and immunologic data from a multinational collaboration.
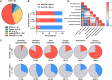
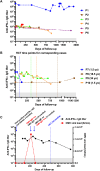
Results: Forty-eight patients (60.0%) had positive anti-IFN-α at baseline; these patients were typically older at time of testing, fulfilled the phenotype of delayed-onset combined immunodeficiency with granuloma and/or autoimmunity (70.8% vs 31.3%, P = .001), and had a history of more frequent viral infections, mainly from the Herpesviridae family (62.5% vs 21.9%, P < .001). These patients also showed higher levels of serum immunoglobulins and expanded populations of peripheral blood autoreactive-prone (CD19hiCD21lo) (14.3 vs 5.2%, P = .016) and double-negative (IgD-CD27-) B cells (12.8 vs 5.8%, P = .001). In cases with longitudinal evaluation, anti-IFN-α titers were largely stable, although an increase was observed with concurrent active cytomegalovirus infections. Despite some decline after transplantation, these autoantibodies persisted during follow-up.
Conclusions: Anti-IFN-α autoantibodies reflect immune dysregulation in partial RAG deficiency. Their production is likely aggravated by environmental factors, especially frequent viral infections. Further studies are needed to define their pathogenic role in RAG deficiency.
Keywords: RAG deficiency; anti–IFN-α autoantibodies; viral infection.
Conflict of interest statement
L.D.N. is supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, 10.13039/100000002National Institutes of Health (NIH, grant ZIA AI001222). J.E.W. is supported by the 10.13039/100000060National Institute of Allergy and Infectious Diseases, National Institutes of Health 5K08AI103035, sub-R01AI100887-05 and R01AI153830-05, Robert A. Good Endowment at 10.13039/100008900University of South Florida, and 10.13039/100001245Jeffrey Modell Foundation. L.I.G.-G. is supported by the Instituto de Salud Carlos III (ISCIII) through project FIS-PI21/01642, cofunded by the 10.13039/501100000780European Union. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Research laboratory studies were performed on deidentified samples under institutional review board–approved protocols at the University of South Florida (USF-Pro00035468, USF-Pro00025693), and Johns Hopkins Medical Institute/Johns Hopkins All Children’s Hospital (JHMI-IRB00175372). Disclosure of potential conflict of interest: J. E. Walter reports receiving grant, research, and/or clinical trial support from Takeda, Janssen, Chiesi, MustangBio, ADMA Biologicals, and Octapharma; serving on consultant and/or advisory boards for Takeda, X4-Pharmaceuticals, CSL-Behring, Grifols, ADMA Biologicals, Enzyvant, and Regeneron; and serving on the speakers’ bureau for Takeda. The rest of the authors declare that they have no relevant conflicts of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials

