Contemporary medical therapy, sex-specific characteristics, and outcomes of patients with non-ischemic cardiomyopathy: a prespecified interim analysis of the BIO-LIBRA study
- PMID: 40697957
- PMCID: PMC12281065
- DOI: 10.1016/j.eclinm.2025.103337
Contemporary medical therapy, sex-specific characteristics, and outcomes of patients with non-ischemic cardiomyopathy: a prespecified interim analysis of the BIO-LIBRA study
Abstract
Background: Contemporary data on characteristics, medical therapy, and outcomes in non-ischemic cardiomyopathy (NICM) with implantable cardioverter defibrillator (ICD) or cardiac resynchronization therapy defibrillator (CRT-D) are lacking, and the role of sex remains unexplored due to historical low enrollment of females in device trials. The purpose of this pre-specified interim analysis of the BIO-LIBRA study was to assess characteristics, medical therapy, and ventricular tachyarrhythmias (VT/VF) or mortality at 12 months in NICM patients with ICD or CRT-D, by sex.
Methods: In this multicenter, prospective, registry study, we recruited patients with primary prevention ICD or CRT-Ds with Home Monitoring®, aiming for 40% female enrollment, utilizing specific recruitment tools. Patients were assessed for the primary endpoint of device-treated VT or VF every 6 months. This study is registered with ClinicalTrials.gov (NCT03884608).
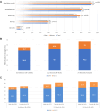
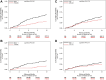
Findings: From May 9, 2019 to October 1, 2021, we enrolled 1000 patients, including 475 (47.5%) females, 30.4% non-white, and 9.2% Hispanic/Latino patients. Beta-blockers were prescribed in 92%, ACE-Inhibitor/ARB in 80%, ARNI in 39%, and SGLT2 in 6% at enrollment with increase in SGLT2 use over time. Diuretics were more frequently prescribed in females. Females had a 52% lower risk of VT/VF or death as compared to men through 12 months (HR = 0.49, 95% CI: 0.31-0.78, p = 0.003), driven by a 54% lower risk of VT/VF (HR = 0.46, 95% CI: 0.25-0.85, p = 0.003), even after adjustments for LVEF or QRS duration. No significant differences were reported by ICD vs. CRT-D.
Interpretation: In a contemporary cohort of patients with NICM and ICD/CRT-D, we report an increased enrollment of females and minorities, an increase in the use of novel guideline-directed medical therapy (GDMT) over time, and a lower risk of ventricular arrhythmias or death in females as compared to men at one year.
Funding: BIOTRONIK Inc.
Keywords: Cardiac resynchronization therapy; Implantable cardioverter defibrillator; Left ventricular dysfunction; Sex differences; Ventricular tachyarrhythmias.
© 2025 Published by Elsevier Ltd.
Conflict of interest statement
Valentina Kutyifa MD, PhD: research grants from Boston Scientific, BIOTRONIK, NIH, Spire Inc., speaker fee from Medtronic, Abbott Medical, consultant fees from Medtronic, Abbott Medical, Biotronik, PIVATAL NIH trial DSMB, AHA CSSP committee. Luigi Di Biase MD, PhD: consultant for Medtronic, Abbott Medical, Biotronik, Zoll, Boston Scientific, Baylis, Zoll, Biosense Webster, Stereotaxis, I rhythm, Zoll, Atricure and has received speaker honoraria/travel from Medtronic, Abbott Medical, Biotronik, Zoll, Boston Scientific, Baylis, Zoll, Biosense Webster, Stereotaxis, I rhythm, Zoll, Atricure and travel support from Medtronic, Abbott Medical, Biotronik, Zoll, Boston Scientific, Baylis, Zoll, Biosense Webster, Stereotaxis, I rhythm, Zoll, Atricure. Karthik Venkatesh Prasad MD: none. Vilma Torres MD: received consultant fees from Biotronik. Aaron Hesselson MD: payment or honoraria from Medtronic and Abbott. Craig J. McCotter MD: none. Gregory Harris MD: received speaker honoraria/travel from Biotronik. Karlene Cox BS–employee of Biotronik Inc. Susan Schleede MS: none. Kevin Heist MD, PhD: consultant: Biotronik, Boston Scientific, and HRS Membership Committee Chair. Scott McNitt MS: none. Mary W. Brown MS: none. Crystal Miller MS–employee of Biotronik Inc. Christopher A. Beck PhD: grants from Boston Scientific, BIOTRONIK, NIH. Jeanne Poole MD–Research contracts direct to institution from: Biotronik, Boston Scientific, AtriCure, Kestra Medical.
Figures
References
-
- Epstein A.E., DiMarco J.P., Ellenbogen K.A., et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines and the heart rhythm society. J Am Coll Cardiol. 2013;61:e6–e75. - PubMed
-
- Al-Khatib S.M., Stevenson W.G., Ackerman M.J., et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the heart rhythm society. Heart Rhythm. 2018;15(10):e73–e189. doi: 10.1016/j.hrthm.2017.10.036. - DOI - PubMed
-
- Yancy C.W., Jessup M., Bozkurt B., et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–1852. - PubMed
-
- Tracy C.M., Epstein A.E., Darbar D., et al. 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg. 2012;144:e127–e145. - PubMed
-
- Epstein A.E., DiMarco J.P., Ellenbogen K.A., et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American heart association task force on practice guidelines (writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices) developed in collaboration with the American association for thoracic surgery and society of thoracic surgeons. J Am Coll Cardiol. 2008;51:e1–e62. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

