Exploring GPR120/FFAR4 pharmacology: Unveiling novel therapeutic avenues through molecular signaling pathways for Alzheimer's disease intervention
- PMID: 40697975
- PMCID: PMC12281026
- DOI: 10.1016/j.bbih.2025.101061
Exploring GPR120/FFAR4 pharmacology: Unveiling novel therapeutic avenues through molecular signaling pathways for Alzheimer's disease intervention
Abstract
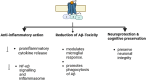
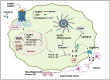
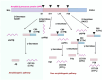
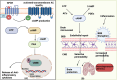
G-protein-coupled receptors (GPCRs) are a major class of membrane proteins involved in numerous physiological and pathological processes. Among them, free fatty acid receptor 4 (FFAR4/GPR120), activated by long-chain free fatty acids, has shown anti-inflammatory effects and is expressed in the brain-implicating its role in neurodegenerative diseases like Alzheimer's disease (AD). AD is characterized by brain atrophy, cognitive decline, and neuroinflammation, involving complex signaling networks. This review explores the pharmacological relevance of GPR120/FFAR4 in AD, focusing on its involvement in neuroinflammatory, amyloidogenic, and intracellular signaling cascades. Targeting GPR120 may help modulate chronic inflammation and amyloid-β accumulation. Additionally, activation of nuclear receptors and regulation of pathways such as MAPK, NLRP3, PPARs, and cAMP have shown promise in mitigating AD pathology. Despite the complexity of brain signaling, GPR120 emerges as a compelling multitarget therapeutic receptor. These insights provide a foundation for developing novel anti-inflammatory strategies in AD treatment.
Keywords: GPCR; MAPK; NLRP3; Neuroinflammation; Neuroprotection; PPARs; Therapeutic targets.
© 2025 The Authors.
Conflict of interest statement
Authors declare no conflict of interest.
Figures






References
-
- Aarti S., Priyanka B. Review on therapeutic effects mediated by omega-3 fatty acids in alzheimer's disease. Asian J. Pharmaceut. Clin. Res. 2018;11(2):54. doi: 10.22159/ajpcr.2018.v11i2.22435. - DOI
-
- Adler B.L., Yarchoan M., Hwang H.M., Louneva N., Blair J.A., Palm R., Smith M.A., Lee H., Arnold S.E., Casadesus G. Neuroprotective effects of the amylin analogue pramlintide on Alzheimer's disease pathogenesis and cognition. Neurobiol. Aging. 2014;35(4):793–801. doi: 10.1016/j.neurobiolaging.2013.10.076. - DOI - PubMed
-
- Ahmed M.R., Jayakumar M., Ahmed M.S., Zamaleeva A.I., Tao J., Li E.H., Job J.K., Pittenger C., Ohtsu H., Rajadas J. Pharmacological antagonism of histamine H2R ameliorated L-DOPA–induced dyskinesia via normalization of GRK3 and by suppressing FosB and ERK in PD. Neurobiol. Aging. 2019;81:177–189. doi: 10.1016/j.neurobiolaging.2019.06.004. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

