Evaluation of the Lodestar DX: a comparison with traditional culture for the detection of bacteriuria in the workup of urinary tract infection
- PMID: 40698006
- PMCID: PMC12280326
- DOI: 10.1093/jacamr/dlaf128
Evaluation of the Lodestar DX: a comparison with traditional culture for the detection of bacteriuria in the workup of urinary tract infection
Abstract
Background: The Lodestar DX is a point-of-care test (POCT) using loop-mediated isothermal amplification (LAMP) to detect common uropathogens. Novel POCTs may provide an adjunct in the diagnostic work up of urinary tract infection (UTI).
Objectives: To evaluate the performance of the Lodestar DX in the identification of bacteriuria.
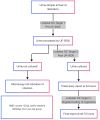
Methods: We compared the Lodestar DX against urine culture results from August to November 2024 at the Eastern Pathology Alliance in non-pregnant adults.
Results: 379 urine samples were tested on the Lodestar DX. 344 (90%) of these passed both internal controls. Summary Lodestar DX performance was sensitivity 85.6% (95% CI 69.2%-94.6%), specificity 92.0% (95% CI 88.1%-94.9%), positive predictive value 64.1% (95% CI 48.7%-77.2%), negative predictive value 97.0% (95% CI 94.0%-98.6%) and overall accuracy 91.2% (95% CI 87.5%-94.0%).In cultures positive for their respective organisms, the sensitivity was 87.9% (n = 66, 95% CI 77.5%-94.6%) for Escherichia coli, 80% (n = 20, 95% CI 56.3%-94.3%) for Enterococcus, 27.3% (n = 11, 95% CI 6.0%-61%) for Staphylococcus aureus, 92.3% (n = 13, 95% CI 64.0%-99.8%) for Staphylococcus saprophyticus, 93.8% (n = 16, 95% CI 69.8%-99.8%) for Proteus mirabilis, 84.2% (n = 19, 95% CI 60.4%-96.6%) for Pseudomonas aeruginosa and 100% sensitivity (n = 2, 95% CI 15.8%-100%) for Klebsiella pneumoniae.
Conclusions: In summary, the Lodestar DX had good performance for the detection of uropathogens apart from S. aureus. Further comparison with other POCTs would be beneficial.
© The Author(s) 2025. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures



Similar articles
-
Point-of-care tests for urinary tract infections to reduce antimicrobial resistance: a systematic review and conceptual economic model.Health Technol Assess. 2024 Nov;28(77):1-109. doi: 10.3310/PTMV8524. Health Technol Assess. 2024. PMID: 39644102 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Clinical effectiveness and cost-effectiveness of tests for the diagnosis and investigation of urinary tract infection in children: a systematic review and economic model.Health Technol Assess. 2006 Oct;10(36):iii-iv, xi-xiii, 1-154. doi: 10.3310/hta10360. Health Technol Assess. 2006. PMID: 17014747
-
Laboratory-based molecular test alternatives to RT-PCR for the diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Oct 14;10(10):CD015618. doi: 10.1002/14651858.CD015618. Cochrane Database Syst Rev. 2024. PMID: 39400904
-
Xpert MTB/RIF Ultra assay for tuberculosis disease and rifampicin resistance in children.Cochrane Database Syst Rev. 2022 Sep 6;9(9):CD013359. doi: 10.1002/14651858.CD013359.pub3. Cochrane Database Syst Rev. 2022. PMID: 36065889 Free PMC article.
References
-
- Antimicrobial Stewardship—RightCare UTI Focus Pack dashboard | NHSBSA. https://www.nhsbsa.nhs.uk/access-our-data-products/epact2/dashboards-and... (9 April 2025, date last accessed).
LinkOut - more resources
Full Text Sources
