Operational Feasibility of "TB Concentration & Transport" and "TB DNA Extraction" Kits for Biosafe Dry Sputum Transport for Direct Detection of Drug Resistance Using Line Probe Assay: A Field Study in India
- PMID: 40698032
- PMCID: PMC12282344
- DOI: 10.1093/ofid/ofaf207
Operational Feasibility of "TB Concentration & Transport" and "TB DNA Extraction" Kits for Biosafe Dry Sputum Transport for Direct Detection of Drug Resistance Using Line Probe Assay: A Field Study in India
Abstract
Background: We recently described the utility of the "TB Concentration & Transport" kit for biosafe, ambient temperature transport of dried sputum on Trans-Filter, and the "TB DNA Extraction" kit for DNA extraction from Trans-Filter for the early diagnosis of drug-resistant tuberculosis (TB). This study aimed to assess the feasibility and compatibility of these kits with line probe assay (LPA) under National Tuberculosis Elimination Programme (NTEP) settings.
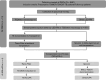
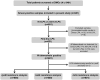
Methods: Patients with presumptive pulmonary TB, multidrug-resistant (MDR) TB, or extensively drug-resistant TB (N = 8491) who attended Designated Microscopy Centers (DMCs, n = 13) under National Reference Laboratories (NRLs) at Bhopal, New Delhi, Chennai, and Bhubaneswar were screened by smear microscopy. The performance of Trans-Filter extracted DNA-based LPA (Kit-LPA) was assessed against Direct-LPA on smear-positive sputum (n = 681), and feedback was obtained from scientists (n = 10) and laboratory technicians (n = 42) regarding logistics, kit usage, training, and troubleshooting. A scoring questionnaire was used to assess (i) the TB Concentration & Transport kit versus conventional sputum transport and (ii) the TB DNA Extraction kit versus Hain GenoLyse kit (statistical significance of the scores was calculated using paired t test).
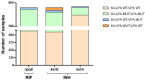
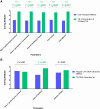
Results: Kit-LPA showed a sensitivity and specificity in the range of 89%-96% and 99%-100%, respectively, for rifampicin and isoniazid resistance detection and was comparable to Direct-LPA (concordance = 99%-100%; κ = 0.94-0.97). Overall scores indicated that (i) sputum transport on Trans-Filters was more convenient as compared to conventional sputum transport (P < .0001) and (ii) Trans-Filter extracted DNA was easily amalgamated with LPA testing for MDR-TB detection.
Conclusions: These findings provide evidence for incorporation of these kits into the NTEP. Their use can facilitate sputum transport from DMCs to NRLs and provide universal drug susceptibility testing to people with TB residing in remote areas.
Keywords: LPA; MDR-TB; biosafe sputum transport; diagnosis; tuberculosis.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. A. Gu. and N. K. G. manufactured the ‘TB Concentration & Transport’ kits, ‘TB DNA extraction’ kits and ‘TBDetect microscopy’ kits and A. Go. participated in the training of laboratory technicians at DMCs and NRLs who used the kits. These authors were not involved in conducting the study and analyzing the results. D. A., R. K. G., V. P. M., A. G., N. K. G., S. H., and J. S. T. are joint inventors in an Indian Provisional Patent application named “Apparatus and method for processing a sample for rapid diagnosis of tuberculosis and safe transport of bacteria” (patent application number 201811042155). All other authors report no potential conflicts of interest.
Figures
Similar articles
-
Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults.Cochrane Database Syst Rev. 2013 Jan 31;(1):CD009593. doi: 10.1002/14651858.CD009593.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2014 Jan 21;(1):CD009593. doi: 10.1002/14651858.CD009593.pub3. PMID: 23440842 Free PMC article. Updated.
-
Rapid molecular tests for tuberculosis and tuberculosis drug resistance: a qualitative evidence synthesis of recipient and provider views.Cochrane Database Syst Rev. 2022 Apr 26;4(4):CD014877. doi: 10.1002/14651858.CD014877.pub2. Cochrane Database Syst Rev. 2022. PMID: 35470432 Free PMC article.
-
Xpert MTB/XDR for detection of pulmonary tuberculosis and resistance to isoniazid, fluoroquinolones, ethionamide, and amikacin.Cochrane Database Syst Rev. 2022 May 18;5(5):CD014841. doi: 10.1002/14651858.CD014841.pub2. Cochrane Database Syst Rev. 2022. PMID: 35583175 Free PMC article.
-
Xpert® MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance.Cochrane Database Syst Rev. 2018 Aug 27;8(8):CD012768. doi: 10.1002/14651858.CD012768.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2021 Jan 15;1:CD012768. doi: 10.1002/14651858.CD012768.pub3. PMID: 30148542 Free PMC article. Updated.
-
Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in HIV-positive adults.Cochrane Database Syst Rev. 2016 May 10;2016(5):CD011420. doi: 10.1002/14651858.CD011420.pub2. Cochrane Database Syst Rev. 2016. PMID: 27163343 Free PMC article.
References
-
- World Health Organization . Global tuberculosis report. 2024. Available at: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/globa.... Accessed 12 April 2024.
-
- World Health Organization . WHO consolidated guidelines on tuberculosis. Module 3: diagnosis—rapid diagnostics for tuberculosis detection 2021 update. 2021. Available at: https://iris.who.int/bitstream/handle/10665/342331/9789240029415-eng.pdf. Accessed 10 May 2024.
-
- National Tuberculosis Elimination Programme . India TB report. 2024. Available at: https://tbcindia.mohfw.gov.in/wp-content/uploads/2024/10/TB-Report_for-W.... Accessed 8 October 2024.
LinkOut - more resources
Full Text Sources
Miscellaneous

