Dihydroartemisinin ameliorates hemarthrosis-induced cartilage degeneration by suppressing chondrocyte senescence via activation of Keap1-Nrf2 signaling pathway
- PMID: 40698064
- PMCID: PMC12282452
- DOI: 10.1016/j.jot.2025.04.006
Dihydroartemisinin ameliorates hemarthrosis-induced cartilage degeneration by suppressing chondrocyte senescence via activation of Keap1-Nrf2 signaling pathway
Abstract
Background: Joint bleeding (hemarthrosis) is a major manifestation of joint trauma, especially repeated and spontaneous in hemophilia patients. Hemarthrosis has been identified to induce the excessive reactive oxygen species (ROS) accumulation and permanent damage in articular cartilage. Dihydroartemisinin (DHA), a well-known clinical anti-malaria drug with few sides effects therapy, has been reported to possess anti-oxidative activity. This study was aimed at exploring the effect of DHA on blood-induced cartilage erosion and its underlying mechanisms.
Methods: Two distinct hemarthrosis models were constructed respectively by fresh blood joint injection in WT and joint needle puncture in F8 -/- mice, and then treated with DHA (10 or 20 mg/kg/day) for 4 weeks. In vitro chondrocytes treated with frozen-thaw blood and DHA (1, 5 or 10 μM) for 24 h. Histopathological, immunofluorescence and western blotting were investigated to demonstrate the effects of DHA on blood-induced chondrocyte senescence, ROS accumulation and extracellular matrix (ECM) degradation. Additionally, Nrf2 inhibitor (MLB385, 30 mg/kg for once a four days) and Nrf2-siRNA were used to investigate the relationship between DHA and Nrf2/Keap1 signaling in vitro and in vivo, respectively.
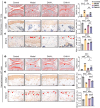
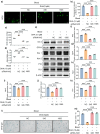
Results: DHA remarkably ameliorated the cartilage degeneration in both two hemarthrosis models. Similarly, in vitro experiments confirmed that DHA promoted the synthesis of ECM in blood-stimulated chondrocytes with a dose-dependent manner. DHA also effectively suppressed blood-induced chondrocyte senescence and ROS accumulation. Mechanistically, DHA activated the Nrf2 signaling by accelerating Keap1 ubiquitination and degradation. Furthermore, Nrf2 siRNA and antagonist abolished the anti-senescence and anti-oxidative functions of DHA, resulting the severe cartilage degeneration in bleeding joint of F8 -/- mice.
Conclusion: Our findings indicate that DHA effectively reduces chondrocyte senescence and mitigates cartilage destruction following hemarthrosis via activation of Nrf2/Keap1 signaling pathway.
The translational potential of this article: On the one hand, this study highlights the important role of chondrocyte senescence in hemarthrosis-induced cartilage degradation, implying that inhibiting chondrocyte senescence may be a viable therapeutic strategy for blood-induced arthropathy. On the other hand, our findings demonstrate the remarkable chondroprotective effect of DHA in bleeding joint by modulating the Nrf2/Keap1 anti-oxidative signaling pathway, suggesting DHA may serve as a potential candidate drug for the therapy of blood-induced arthropathy.
Keywords: Cartilage degeneration; Chondrocyte senescence; Dihydroartemisinin; Hemarthrosis; Nrf2/Keap1 signaling pathway; Oxidative stress.
© 2025 The Authors.
Conflict of interest statement
All authors declare that they have no conflict of interest.
Figures






Similar articles
-
Trigonelline Shields Chondrocytes from Oxidative Damage in Osteoarthritis through Activation of the Keap1/Nrf2/ARE Signaling Pathway.Appl Biochem Biotechnol. 2025 Jul;197(7):4586-4601. doi: 10.1007/s12010-025-05232-1. Epub 2025 May 5. Appl Biochem Biotechnol. 2025. PMID: 40323359
-
Non-clotting factor therapies for preventing bleeds in people with congenital hemophilia A or B.Cochrane Database Syst Rev. 2024 Feb 27;2(2):CD014544. doi: 10.1002/14651858.CD014544.pub2. Cochrane Database Syst Rev. 2024. PMID: 38411279 Free PMC article.
-
Growth factor independence 1 ameliorates osteoarthritis by inhibiting chondrocyte ferroptosis via inactivation of MAPK signaling pathway.J Orthop Translat. 2025 Jul 29;54:101-114. doi: 10.1016/j.jot.2025.07.003. eCollection 2025 Sep. J Orthop Translat. 2025. PMID: 40787173 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
ZC3H15 suppression ameliorates bone cancer pain through inhibiting neuronal oxidative stress and microglial inflammation.Neoplasia. 2025 Mar;61:101123. doi: 10.1016/j.neo.2025.101123. Epub 2025 Feb 4. Neoplasia. 2025. PMID: 39908779 Free PMC article.
References
-
- Gelber A.C., Hochberg M.C., Mead L.A., Wang N.Y., Wigley F.M., Klag M.J. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann Intern Med. 2000;133(5):321–328. - PubMed
-
- Jansen N.W., Roosendaal G., Bijlsma J.W., Degroot J., Lafeber F.P. Exposure of human cartilage tissue to low concentrations of blood for a short period of time leads to prolonged cartilage damage: an in vitro study. Arthritis Rheum. 2007;56(1):199–207. - PubMed
-
- Tajima T., Yoshida E., Yamashita A., Ohmura S., Tomitaka Y., Sugiki M., et al. Hemoglobin stimulates the expression of matrix metalloproteinases, MMP-2 and MMP-9 by synovial cells: a possible cause of joint damage after intra-articular hemorrhage. J Orthop Res. 2005;23(4):891–898. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

