CMD-OPT model enables the discovery of a potent and selective RIPK2 inhibitor as preclinical candidate for the treatment of acute liver injury
- PMID: 40698146
- PMCID: PMC12278411
- DOI: 10.1016/j.apsb.2025.05.003
CMD-OPT model enables the discovery of a potent and selective RIPK2 inhibitor as preclinical candidate for the treatment of acute liver injury
Abstract
Acute liver injury (ALI) serves as a critical precursor and major etiological factor in the progression and ultimate manifestation of various hepatic disorders. The prevention and treatment of ALI is still a serious global challenge. Given the limited therapeutic options for ALI, exploring novel targeted therapeutic agents becomes imperative. The potential therapeutic efficacy of inhibiting RIPK2 is highlighted, as it may provide significant benefits by attenuating the MAPK pathway and NF-κB signaling. Herein, we propose a CMD-OPT model, a two-stage molecular optimization tool for the rapid discovery of RIPK2 inhibitors with optimal properties. Compound RP20, which targets the ATP binding site, demonstrated excellent kinase specificity, ideal oral pharmacokinetics, and superior therapeutic effects in a model of APAP-induced ALI, positioning RP20 as a promising preclinical candidate. This marks the first application of RIPK2 inhibitors in ALI treatment, opening a novel therapeutic pathway for clinical applications. These results highlight the efficacy of the CMD-OPT model in producing lead compounds from known active molecules, showcasing its significant potential in drug discovery.
Keywords: Acute liver injury; Anti-inflammatory; CMD-OPT; Candidate; Co-crystal; Drug discovery; Kinase specificity; RIPK2 inhibitors.
© 2025 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




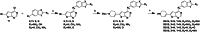





Similar articles
-
Development of Thieno[3,2-d]pyrimidine derivatives as potent RIPK2 inhibitors with Prominent In vitro and In vivo anti-inflammatory efficacy.Eur J Med Chem. 2025 Nov 5;297:117932. doi: 10.1016/j.ejmech.2025.117932. Epub 2025 Jul 5. Eur J Med Chem. 2025. PMID: 40639288
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

