Organic probes for NO-activatable biomedical imaging: NIR fluorescence, self-luminescence, and photoacoustic imaging
- PMID: 40698164
- PMCID: PMC12278105
- DOI: 10.1039/d5sc03611a
Organic probes for NO-activatable biomedical imaging: NIR fluorescence, self-luminescence, and photoacoustic imaging
Abstract
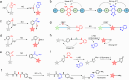
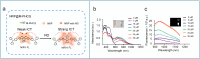
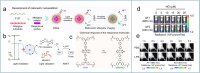
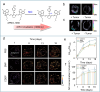
Nitric oxide (NO) is a crucial signaling molecule involved in diverse physiological and pathological processes, making its precise detection essential for exploring its biological roles. Optical imaging is particularly attractive for NO detection due to its non-invasive nature, high sensitivity, and excellent spatial resolution. However, it suffers from limited tissue penetration and low signal-to-background ratios resulting from strong light scattering and autofluorescence. To overcome these challenges, several advanced imaging strategies have been developed, including near-infrared (NIR) fluorescence imaging that leverages optical regions with less light-tissue interactions, self-luminescence imaging that avoids the need for real-time light excitation, and photoacoustic imaging that detects acoustic signals with minimal attenuation. This review systematically summarizes recent advances in organic molecular probes for NO detection using these imaging modalities, focusing on their design strategies, recognition mechanisms, and biological applications. Finally, current challenges and future directions are discussed to guide the development of next-generation NO probes for both fundamental research and clinical translation.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












Similar articles
-
Near-Infrared-II Fluorescent Probes for Analytical Applications: From In Vitro Detection to In Vivo Imaging Monitoring.Acc Chem Res. 2025 Feb 18;58(4):543-554. doi: 10.1021/acs.accounts.4c00671. Epub 2025 Feb 5. Acc Chem Res. 2025. PMID: 39907648 Review.
-
Single- and multi-modal molecular probes with second near-infrared activatable optical signals for disease diagnosis and theranostics.Chem Soc Rev. 2025 Aug 11;54(16):7561-7609. doi: 10.1039/d5cs00502g. Chem Soc Rev. 2025. PMID: 40693322 Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Surveillance of Barrett's oesophagus: exploring the uncertainty through systematic review, expert workshop and economic modelling.Health Technol Assess. 2006 Mar;10(8):1-142, iii-iv. doi: 10.3310/hta10080. Health Technol Assess. 2006. PMID: 16545207
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

