Real-world Evidence from a Retrospective Multicentre Analysis on First-line Therapy for Metastatic Papillary Renal Cell Carcinoma. A GUARDIANS Project
- PMID: 40698263
- PMCID: PMC12280342
- DOI: 10.1016/j.euros.2025.06.011
Real-world Evidence from a Retrospective Multicentre Analysis on First-line Therapy for Metastatic Papillary Renal Cell Carcinoma. A GUARDIANS Project
Abstract
Background and objective: Papillary renal cell carcinoma (pRCC) is a rare disease. The optimal treatment of metastatic pRCC is still unclear. We evaluated real-world treatment outcomes of first-line treatment in this cohort in Germany.
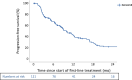
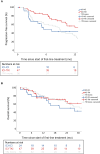
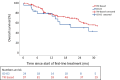
Methods: Patients with advanced or metastatic pRCC were eligible. Adverse events (AEs) were reported according to Common Terminology Criteria for Adverse Events version 5.0. The overall response rate was accessed according to the local standard. Progression-free survival (PFS) was calculated from the start of treatment to progression or death. Descriptive statistics and Kaplan-Meier plots were utilised, where appropriate.
Key findings and limitations: In total, 121 suitable patients (77% male) with a median age of 63 yr (quartiles 55, 70) were included. Prior nephrectomy was performed in 78%. Eastern Cooperative Oncology Group performance status 0-1 was reported in 74%. Lymphatic (68%) and pulmonary (42%) metastases were most common. Of the patients, 59% received first-line immune checkpoint inhibitor (ICI) combination therapies (ICI-ICI: 20%, tyrosine kinase inhibitor [TKI]-ICI: 39%), and 41% of patients received TKI monotherapy, predominantly sunitinib. The median follow-up time was 33.3 mo (interquartile range 14.8-46.7). The median PFS was 5.4 mo (95% confidence interval [CI]: 3.2-7.6) for ICI-ICI combinations, 16.9 mo (95% CI: 7.2-26.6) for ICI-TKI combinations, and 8.8 mo (95% CI: 7.0-10.7) for TKI monotherapy. Of all the patients, 70% and 35% experienced all-grade and grade 3-5 AEs, respectively. AEs of any cause led to discontinuation in 33% of patients.
Conclusions and clinical implications: TKI-based therapies are applied frequently in pRCC patients. Our data support the use of ICI plus TKI as a first-line standard for patients with pRCC. The major limitations were the retrospective data capture and short follow-up of our study. Additional analyses to tailor treatment strategies in patients with metastatic pRCC are warranted.
Patient summary: In this report, we looked at the outcome of first-line treatment of patients with metastatic papillary renal cell cancer (pRCC). Tyrosine kinase inhibitor (TKI)-based therapies are applied frequently in pRCC. Our data support the use of immune checkpoint inhibitor plus TKI as a first-line standard for patients with pRCC. However, further studies are needed to optimise treatment in patients with metastatic pRCC.
Keywords: First-line treatment; Immune checkpoint inhibitor; Papillary renal cell cancer; Tyrosine kinase inhibitor.
© 2025 The Author(s).
Figures
References
-
- Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
LinkOut - more resources
Full Text Sources

