Prediction of Atrial Fibrillation Using Radiomic Features of Left Atrial Epicardial Adipose Tissue on Noncontrast Cardiac Computed Tomography
- PMID: 40698311
- PMCID: PMC12277846
- DOI: 10.1016/j.cjco.2025.03.024
Prediction of Atrial Fibrillation Using Radiomic Features of Left Atrial Epicardial Adipose Tissue on Noncontrast Cardiac Computed Tomography
Abstract
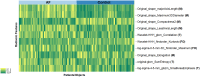
Background: Early detection of atrial fibrillation (AF) can prevent AF-related complications. Radiomic analysis of epicardial adipose tissue (EAT) was shown to predict AF recurrence postablation, but only limited data exist regarding left atrial EAT (LA-EAT) radiomic analysis for predicting AF in patients with yet unknown AF. Our aim was to develop prediction model for AF, based on the association of machine learning-based radiomic analysis of LA-EAT and AF.
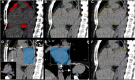
Methods: Retrospective matched case-control study of patients with and without AF, undergoing noncontrast electrocardiographic (ECG)-gated cardiac computed tomography (CT). Segmentation of LA-EAT and extraction of LA-EAT radiomic features were performed using syngo.via Frontier (Siemens Healthineers, Forchheim, Germany). Univariate analysis identified radiomic features associated with AF. Predictive models for AF were developed via logistic regression and machine learning-based random forest analyses. Models were validated on external cohort of patients with 1:1 AF : control ratio and deployed in a real-world setting with an AF : control ratio of 15:85.
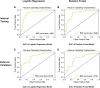
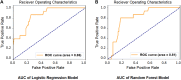
Results: The study included 280 patients, 120 with documented AF and 160 matched controls. Based on LA-EAT radiomic features, which were significantly associated with AF, logistic regression and random forest models were constructed and tested on separate internal cohort of patients, yielding area under the curve (AUC) of 0.88 and 0.86, respectively, for prediction of AF. External validation verified these results (AUC 0.84 and 0.78, respectively). Both models were further validated in a real-world setting cohort (AUC 0.85 and 0.81, respectively).
Conclusions: Models, based on LA-EAT radiomic features extracted from noncontrast ECG-gated cardiac CT, could accurately predict AF, suggesting a potential widespread noninvasive method for predicting the presence of AF.
Clinical registration number: 0281-23-ASF.
Contexte: La détection précoce de la fibrillation auriculaire (FA) peut prévenir les complications qui y sont associées. L’analyse radiomique du tissu adipeux épicardique (TAE) a déjà démontré son utilité pour prédire la récidive de FA après ablation, mais il n'existe que peu de données concernant l'analyse radiomique du tissu adipeux de l'oreillette gauche (TAE-OG) pour prédire la FA chez les patients sans diagnostic préalable de FA. Notre objectif était de développer un modèle de prédiction de la FA, basé sur l'association de la FA avec l'analyse radiomique du TAE-OG assistée par apprentissage automatique.
Méthodologie: Étude rétrospective cas-témoins appariés incluant des patients avec et sans FA, soumis à une tomodensitométrie cardiaque sans contraste, avec synchronisation ECG. La segmentation du TAE-OG et l'extraction des caractéristiques radiomiques du TAE-OG ont été réalisées à l'aide du logiciel syngo.via Frontier (Siemens Healthineers, Forchheim, Allemagne). Une analyse univariée a permis d'identifier les caractéristiques radiomiques associées à la FA. Des modèles prédictifs de la FA ont été développés par régression logistique et par des analyses de forêt aléatoire basées sur l'apprentissage automatique. Les modèles ont été validés sur une cohorte externe de patients avec un ratio AF:contrôle de 1:1 et déployés dans un environnement réel avec un ratio AF:contrôle de 15:85.
Résultats: L'étude a porté sur 280 patients, dont 120 avec une FA documentée et 160 témoins appariés. Sur la base des caractéristiques radiomiques du TAE-OG qui étaient significativement associées à la FA, des modèles de régression logistique et par forêt aléatoire ont été construits et testés sur une cohorte interne de patients distincte, produisant une aire sous la courbe (ASC) de 0,88 et 0,86, respectivement, pour la prédiction de la FA. Une validation externe a confirmé ces résultats (ASC de 0,84 et 0,78, respectivement). Les deux modèles ont été validés dans une cohorte de patients en conditions réelles (AUC de 0,85 et 0,81, respectivement).
Conclusions: Les modèles, basés sur les caractéristiques radiomiques du TAE-OG extraites de la tomodensitométrie cardiaque sans contraste, avec synchronisation ECG, pouvaient prédire avec précision la FA, ce qui suggère une méthode non invasive potentiellement applicable à grande échelle pour prédire la présence de FA.
© 2025 The Authors.
Figures

Similar articles
-
Radiomic features of peri-left atrial epicardial adipose tissue and atrial fibrillation recurrence after ablation.Open Heart. 2025 Jul 8;12(2):e003364. doi: 10.1136/openhrt-2025-003364. Open Heart. 2025. PMID: 40628672 Free PMC article.
-
Chest CT-based analysis of radiomic and volumetric differences in epicardial adipose tissue in HFrEF patients with and without AF.BMC Cardiovasc Disord. 2025 Aug 7;25(1):588. doi: 10.1186/s12872-025-05056-1. BMC Cardiovasc Disord. 2025. PMID: 40770297 Free PMC article.
-
The Relationship Between Cardiac CT-based Left Atrial Structure and Epicardial Adipose Tissue and Postablation Atrial Fibrillation Recurrence Within 2 Years.J Thorac Imaging. 2024 Nov 1;39(6):351-358. doi: 10.1097/RTI.0000000000000789. Epub 2024 May 27. J Thorac Imaging. 2024. PMID: 38800955
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
-
Relationship between epicardial adipose tissue volume and atrial fibrillation : A systematic review and meta-analysis.Herz. 2016 Aug;41(5):421-7. doi: 10.1007/s00059-015-4387-z. Epub 2015 Dec 10. Herz. 2016. PMID: 26659845 English.
References
-
- Lloyd-Jones D.M., Wang T.J., Leip E.P., et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. - PubMed
-
- Hindricks G., Potpara T., Dagres N., et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC). Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. - PubMed
-
- Menezes A.R., Lavie C.J., DiNicolantonio J.J., et al. Atrial fibrillation in the 21st century: a current understanding of risk factors and primary prevention strategies. Mayo Clin Proc. 2013;88:394–409. - PubMed
LinkOut - more resources
Full Text Sources

