Pre-Exposure to Chemicals Increases Springtail Vulnerability to High Temperatures
- PMID: 40698595
- PMCID: PMC12284914
- DOI: 10.1111/gcb.70374
Pre-Exposure to Chemicals Increases Springtail Vulnerability to High Temperatures
Abstract
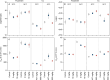
Global climate change is increasing the frequency and intensity of heat waves, posing a significant threat to ectothermic organisms. Concurrently, chemical pollution, including heavy metals and pesticides, remains a pervasive environmental stressor. This study investigates the effects of sub-lethal copper and fluazinam exposure on the thermal tolerance of the soil-dwelling springtail, Folsomia candida. Using a thermal death time (TDT) framework, we assessed how pre-exposure to these toxicants at two acclimation temperatures (20°C and 24°C) influenced survival under heat stress. Our findings indicate that toxicant exposure reduced heat tolerance at moderately high temperatures (32.5°C) but had negligible effects at extreme temperatures (37°C). Acclimation at 24°C mitigated the negative effects of both toxicants, suggesting an enhanced capacity for cellular homeostasis under warm conditions. Additionally, soil type influenced thermal tolerance, highlighting the importance of environmental context in multiple stressor interactions. These findings highlight the need to integrate realistic thermal exposure scenarios in ecotoxicological assessments to improve predictions of organismal vulnerability under climate change.
Keywords: Folsomia candida; Collembola; climate change; multiple stressors; pesticides; soil arthropods; thermal death time; thermal stress.
© 2025 The Author(s). Global Change Biology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Bowler, K. 2018. “Heat Death in Poikilotherms: Is There a Common Cause?” Journal of Thermal Biology 76: 77–79. - PubMed
-
- Cossins, A. R. , and Bowler K.. 1987. Temperature Biology of Animals. Chapman and Hall.
-
- Dallas, H. F. , and Rivers‐Moore N. A.. 2012. “Critical Thermal Maxima of Aquatic Macroinvertebrates: Towards Identifying Bioindicators of Thermal Alteration.” Hydrobiologia 679, no. 1: 61–76. 10.1007/s10750-011-0856-4. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

