Brain atrophy and associations with long-term disability and cognitive function in participants with relapsing multiple sclerosis treated with ozanimod: Results from phase 3 and open-label extension trials
- PMID: 40698614
- PMCID: PMC12432276
- DOI: 10.1177/13524585251355842
Brain atrophy and associations with long-term disability and cognitive function in participants with relapsing multiple sclerosis treated with ozanimod: Results from phase 3 and open-label extension trials
Abstract
Background: In phase 3 trials, ozanimod reduced brain atrophy and improved cognitive processing speed compared with interferon β-1a (IFN) in participants with relapsing multiple sclerosis (RMS).
Objectives: To assess long-term brain volume changes and associations with clinical/cognitive outcomes during an open-label extension ([OLE] DAYBREAK [NCT02576717]).
Methods: Completers of phase 3 "parent" trials were eligible to receive ozanimod 0.92 mg in DAYBREAK. Whole brain, thalamic, and cortical gray matter volumes (WBV, TV, and CGMV, respectively) were analyzed annually.
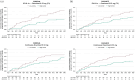
Results: Participants receiving continuous ozanimod had sustained, low rates of WBV loss through OLE month (M)60 (annualized least-squares mean percent change from parent baseline: RADIANCE, -0.27; SUNBEAM, -0.35). Compared with participants switched from IFN, these participants had lower reductions in WBV (parent baseline through OLE M48 [RADIANCE] and OLE M60 [SUNBEAM]). Larger baseline brain volumes were associated with numerically better Symbol Digit Modalities Test scores and lower 3-month confirmed disability progression (CDP) incidence. Annualized TV atrophy ⩽1.0% was associated with lower 3-month CDP.
Conclusion: This study confirms the sustained efficacy of ozanimod in reducing brain atrophy rates for up to 7 years. Brain volume preservation was associated with faster cognitive processing speed and slower physical disability progression.
Keywords: MRI; Multiple sclerosis; Symbol Digit Modalities Test; confirmed disability progression; sphingosine 1-phosphate receptor modulators; thalamus.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J.A.C.: personal compensation for consulting for Astoria, Atara, Biogen, Bristol Myers Squibb, Convelo, and Viatris. D.L.A.: consulting fees from Biogen, Biohaven, Bristol Myers Squibb, Eli Lilly, EMD Serono, Find Therapeutics, Frequency Therapeutics, GlaxoSmithKline, Idorsia Pharmaceuticals, Kiniksa Pharmaceuticals, Merck, Novartis, Race to Erase MS, Roche, Sanofi-Aventis, Shionogi, and Xfacto Communications and equity interest in NeuroRx. J.D.: personal compensation for consulting from Biogen, Bristol Myers Squibb, Janssen Pharmaceuticals, and Novartis; speaker for Biogen, Consortium of MS Centers, and EMD Serono; and grant funding from Biogen, Bristol Myers Squibb, Canadian MS Society, Consortium of MS Centers, EMD Serono, Genentech, National Institutes of Health, and National MS Society. H.-P.H.: personal fees for consulting, serving on steering committees, and speaking from Bayer Healthcare, Biogen, Celgene, GeNeuro, Genzyme, MedImmune, Merck, Novartis, Octapharma, Roche, Sanofi, and TG Therapeutics. L.K.: received no personal compensation. His institutions (University Hospital Basel/Stiftung Neuroimmunology and Neuroscience Basel) received payments for steering committee, advisory and data safety monitoring board participation, consultancy services, and educational activities from Bayer, Biogen, Bristol Myers Squibb, Celltrion, Clene Nanomedicine, EMD Serono Research and Development, Galapagos, Genentech, Immunic, Janssen, Kiniksa Pharmaceuticals, Laboratoires Juvise Pharmaceuticals, Merck Healthcare, Merck Sharp & Dohme, Minoryx Therapeutics, Neurostatus-UHB, Novartis, Roche, Sanofi, Shionogi, Wellmera, and Zai Lab and research support from Novartis, Roche, and Innosuisse. G.C.: compensation for consulting and/or speaking activities from Almirall, Biogen, Celgene, EXCEMED, Forward Pharma, Genzyme, Merck, Novartis, Roche, Sanofi, and Teva. K.S.: consulting for Biogen, Celgene, Genzyme, Merck, Novartis, Ono Pharma, Roche, Synthon, and Teva. L.S.: consulted for AbbVie, Atreca, Bristol Myers Squibb, EMD Serono, Novartis, Pasithea, Teva, TG Therapeutics, and 180 Life Sciences and research support from Atara and Bristol Myers Squibb. A.B.-O.: personal fees for advisory board participation and/or consulting from Abata, Accure, Atara Biotherapeutics, Biogen, BMS/Celgene/Receptos, GlaxoSmithKline, Gossamer, Horizon Therapeutics, Immunic, Janssen/Actelion, MedImmune, Merck/EMD Serono, Novartis, Roche/Genentech, Sangamo, Sanofi-Genzyme, and Viracta and grant support to the University of Pennsylvania from Biogen, Merck/EMD Serono, Novartis, and Roche/Genentech. X.M.: Professor Montalban’s institution has received compensation for lecture honoraria and travel expenses, participation in scientific meetings, clinical trial steering committee membership, or clinical advisory board participation in recent years from AbbVie, Actelion, Alexion, AstraZeneca, Autolus, Bial PD, Biogen, Bristol Myers Squibb/Celgene, EMD Serono, Genzyme, Hoffmann-La Roche, Immunic Therapeutics, Indivi, Janssen Pharmaceuticals, Juvisé Pharmaceutical, Lilly, MedDay, Medscape, Merck, Merz Therapeutics, Mylan-Viatris, Nervgen, Neuraxpharm, Novartis, Peervoice, Rewind Therapeutics, Samsung-Biosys, Sandoz, Sanofi-Genzyme, Teva Pharmaceuticals, TG Therapeutics, Zenas Biopharma, Excemed, ECTRIMS, MSIF, and NMSS or any of their affiliates. E.K.H.: honoraria/research support from Biogen, Merck Serono, Novartis, Roche, and Teva; has served as a member of advisory boards for Actelion, Biogen, Celgene, Merck Serono, Novartis, and Sanofi-Genzyme; and support from the Czech Ministry of Education—project Cooperatio LF1, research area Neuroscience, and the project National Institute for Neurological Research (Program EXCELES, ID project No LX22NPO5107)—funded by the European Union-Next Generation EU. J.K.S.: former employee of Bristol Myers Squibb. C.P., C.Y.C., and J.V.R.: employees and/or shareholders of Bristol Myers Squibb. B.A.C.C.: personal compensation for consulting from Alexion, Atara, Autobahn, Avotres, Biogen, Boston Pharma, EMD Serono, Gossamer Bio, Hexal/Sandoz, Horizon, Immunic AG, Kyverna, Neuron23, Novartis, Sanofi, Siemens, and TG Therapeutics and research support from Genentech and Kyverna.
Figures




References
-
- Filippi M, Bar-Or A, Piehl F, et al. Multiple sclerosis. Nat Rev Dis Primers 2018; 4: 43. - PubMed
-
- Thompson AJ, Baranzini SE, Geurts J, et al. Multiple sclerosis. Lancet 2018; 391: 1622–1636. - PubMed
-
- De Stefano N, Airas L, Grigoriadis N, et al. Clinical relevance of brain volume measures in multiple sclerosis. CNS Drugs 2014; 28: 147–156. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

