Plasticity in climate change responses
- PMID: 40698780
- PMCID: PMC12586308
- DOI: 10.1111/brv.70056
Plasticity in climate change responses
Abstract
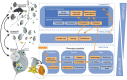
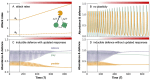
Recent research has shown that climate change can both induce and modulate the expression of plastic traits but our understanding of the role of phenotypic plasticity as an adaptive response to climate change is limited. In this review, we dissect the mechanisms and impact of phenotypic plasticity as a response to accumulating climatic pressures on the individual, species and community levels. (i) We discuss how plasticity can affect individuals, populations and community dynamics and how climate change can alter the role of plasticity. We hypothesise that some pathways to phenotypic plasticity such as irreversible and anticipatory organismal responses will be reduced under increasing climate change. (ii) We then propose an integrated conceptual framework for studying phenotypic plasticity to advance our understanding of the feedbacks between the different levels of biological organisation. (iii) By formulating as yet unaddressed research questions within and across levels of biological organisation, we aim to instigate new research on phenotypic plasticity and its role in climate change responses.
Keywords: climate change; community; ecological feedbacks; microbiome; molecular pathways; phenotypic plasticity; species interactions; warming.
© 2025 The Author(s). Biological Reviews published by John Wiley & Sons Ltd on behalf of Cambridge Philosophical Society.
Figures



References
-
- Abdelrahman, M. , Ishii, T. , El‐Sayed, M. & Tran, L. P. (2020). Heat sensing and lipid reprograming as a signaling switch for heat stress responses in wheat. Plant and Cell Physiology 61, 1399–1407. - PubMed
-
- Allen, A. P. , Gillooly, J. F. & Brown, J. H. (2005). Linking the global carbon cycle to individual metabolism. Functional Ecology 19, 202–213.
-
- Anstey, M. L. , Rogers, S. M. , Ott, S. R. , Burrows, M. & Simpson, S. J. (2009). Serotonin mediates behavioral gregarization underlying swarm formation in desert locusts. Science 323, 627–630. - PubMed
-
- Atkinson, D. (1994). Temperature and organism size ‐ a biological law for ectotherms? Advances in Ecological Research 25, 1–58.
Publication types
MeSH terms
Grants and funding
- 21-29169S/Grant Agency of the Czech Republic
- ANR-19-CE02-0001-01/Agence Nationale de la Recherche
- CEECIND/01526/2018/Fundação para a Ciência e Tecnologia
- PTDC/BIA-BMA/1494/2020/Fundação para a Ciência e Tecnologia
- UIDP/04378/2020/Fundação para a Ciência e Tecnologia
- UUIDB/04378/2020/Fundação para a Ciência e Tecnologia
- LA/P/0140/2020/Fundação para a Ciência e Tecnologia
- MR/V024744/2/UK Research & Innovation
- 511084840/Deutsche Forschungsgemeinschaft
- C16/2017/002/KU Leuven Research Council
- C16/2023/007/KU Leuven Research Council
- NE/Y001184/1/Natural Environment Research Council
LinkOut - more resources
Full Text Sources
Medical

