Kidney Outcomes With Glucagon-Like Peptide-1 Receptor Agonists Versus Other Glucose-Lowering Agents in People With Type 2 Diabetes: A Systematic Review and Meta-Analysis of Real-World Data
- PMID: 40698871
- PMCID: PMC12285216
- DOI: 10.1002/dmrr.70066
Kidney Outcomes With Glucagon-Like Peptide-1 Receptor Agonists Versus Other Glucose-Lowering Agents in People With Type 2 Diabetes: A Systematic Review and Meta-Analysis of Real-World Data
Abstract
Aims: Randomized placebo-controlled clinical trials showed that glucagon-like peptide-1 receptor agonists (GLP-1 RA) reduce kidney risk in patients with type 2 diabetes (T2D), prominently in those with chronic kidney disease. It is unclear whether these findings may apply to broader populations of patients with T2D treated in real-world settings and compared to active controls. We summarised real-world data of adverse kidney outcomes among patients with T2D initiating GLP-1 RA versus other glucose-lowering agents.
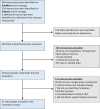
Materials and methods: We searched PubMed and Embase for observational cohort studies (April 2005-January 2025; PROSPERO CRD42023405356). Initiators of GLP-1 RA were compared to sodium-glucose cotransporter-2 inhibitors (SGLT2i), dipeptidyl-peptidase 4 inhibitors (DPP4i), sulfonylureas, or basal insulin. Outcomes included risks of albuminuria progression, ≥ 40 or ≥ 50% eGFR reduction from baseline, acute kidney injury (AKI), kidney-related hospitalizations, and end-stage kidney disease (ESKD), per data availability. We synthesised the data using inverse variance-weighted averages of logarithmic hazard ratios (HR)s in random-effect models.
Results: Thirty-one studies were eligible, encompassing 1,601,389 patients (mean age 49-78 years, 5%-64% women), with 21, 6, 5, and 1 of them using SGLT2i, DPP4i, basal insulin, and sulfonylureas as a comparator, respectively. Compared with SGLT2i, GLP-1 RA initiators had higher risks for AKI (HR [95% CI] 1.12 [1.05-1.20]), kidney-related hospitalizations (1.66 [1.01-2.73]), and ≥ 40% reduction in eGFR (1.40 [1.27-1.53]), without evidence for differences in risks of ≥ 50% eGFR reduction or ESKD. Compared to DPP4i, GLP-1 RA initiators had lower risks for experiencing ≥ 50% eGFR reduction (0.84 [0.76-0.92]), kidney-related hospitalizations (0.73 [0.65-0.83]), and ESKD (0.70 [0.63-0.78]). Similar benefits were observed when comparing GLP-1 RA to sulfonylureas. Compared to basal insulin, GLP-1 RA initiation was associated with a lower risk of albuminuria progression (0.89 [0.80-0.99]), with inconsistent data regarding possible benefits in reducing ESKD risk.
Conclusions: In patients with T2D, initiation of GLP-1 RA in real-world settings may be associated with improved kidney outcomes compared to DPP4i, sulfonylureas, and basal insulin, and worse kidney outcomes compared to SGLT2i.
Keywords: GLP‐1 RA; kidney outcomes; meta‐analysis; real world data; systematic review; type 2 diabetes.
© 2025 The Author(s). Diabetes/Metabolism Research and Reviews published by John Wiley & Sons Ltd.
Conflict of interest statement
A.F. and D.R.S. have no conflict of interest to declare. I.Y. and A.R. received hourly payments from AstraZeneca through Hadassah Medical Centre and from Novo Nordisk. M.S. reports travel support from Novo Nordisk and AstraZeneca through Hadassah Medical Centre and lecturing fees from AstraZeneca (2022). O.M. reports Advisory Board membership for Novo Nordisk, Eli Lilly, Sanofi, MerckSharp and Dohme, Boehringer Ingelheim, AstraZeneca and BOL Pharma, research grant support through Hadassah Hebrew University Hospital from Novo Nordisk and AstraZeneca, and Speaker's Bureau participation for AstraZeneca, Novo Nordisk, Eli Lilly, Sanofi, Merck Sharp and Dohme, and Boehringer Ingelheim. From May 1st, 2023, Ofri Mosenzon has been an employee of Regeneron Pharmaceuticals Inc. G.A.H. reported previous Advisory Board: Sanofi and Eli Lilly. She is a current PI in several RCTs conducted by Novo Nordisk, Eli Lilly, Sanofi, AstraZeneca and Bayer, including the Confidence study, through Hadassah Medical Centre. G.A.H. reports travel support from Novo Nordisk and Medetronic through Hadassah Medical Centre and AstraZeneca through Sheba medical centre. Speakers Bureau: AstraZeneca, Novo Nordisk, Eli Lilly and Sanofi. G.L. reports participation in previous Advisory Boards: Novo Nordisk, Eli Lilly, Sanofi, Merck Sharp and Dohme, Boehringer Ingelheim, AstraZeneca and Medtronic. He reports travel support from Novo Nordisk, Boehringer Ingelheim and AstraZeneca through Hadassah Medical Centre. Speakers Bureau: AstraZeneca, Novo Nordisk, Eli Lilly, Boehringer Ingelheim.
Figures



Similar articles
-
Dipeptidyl peptidase-4 inhibitors, glucagon-like peptide 1 receptor agonists and sodium-glucose co-transporter-2 inhibitors for people with cardiovascular disease: a network meta-analysis.Cochrane Database Syst Rev. 2021 Oct 25;10(10):CD013650. doi: 10.1002/14651858.CD013650.pub2. Cochrane Database Syst Rev. 2021. PMID: 34693515 Free PMC article.
-
Insulin and glucose-lowering agents for treating people with diabetes and chronic kidney disease.Cochrane Database Syst Rev. 2018 Sep 24;9(9):CD011798. doi: 10.1002/14651858.CD011798.pub2. Cochrane Database Syst Rev. 2018. PMID: 30246878 Free PMC article.
-
Comparative Effectiveness of Glucagon-Like Peptide-1 Receptor Agonists, Sodium-Glucose Cotransporter 2 Inhibitors, Dipeptidyl Peptidase-4 Inhibitors, and Sulfonylureas for Sight-Threatening Diabetic Retinopathy.Ophthalmol Retina. 2024 Oct;8(10):943-952. doi: 10.1016/j.oret.2024.05.003. Epub 2024 May 11. Ophthalmol Retina. 2024. PMID: 38735641 Free PMC article.
-
Glucagon-like peptide 1 (GLP-1) receptor agonists for people with chronic kidney disease and diabetes.Cochrane Database Syst Rev. 2025 Feb 18;2(2):CD015849. doi: 10.1002/14651858.CD015849.pub2. Cochrane Database Syst Rev. 2025. PMID: 39963952
-
Risk of bone fracture by using dipeptidyl peptidase-4 inhibitors, glucagon-like peptide-1 receptor agonists, or sodium-glucose cotransporter-2 inhibitors in patients with type 2 diabetes mellitus: a network meta-analysis of population-based cohort studies.Front Endocrinol (Lausanne). 2024 Oct 11;15:1410883. doi: 10.3389/fendo.2024.1410883. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39464183 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

