Age-Associated Cortical Thinning in Speech Motor Regions Precedes Hippocampal Decline: Implications for Alzheimer's Disease
- PMID: 40698900
- PMCID: PMC12284906
- DOI: 10.1002/hbm.70288
Age-Associated Cortical Thinning in Speech Motor Regions Precedes Hippocampal Decline: Implications for Alzheimer's Disease
Abstract
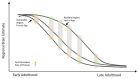
Speech-motor and cognitive impairments are commonly observed in age-related neurodegenerative diseases, including mild cognitive impairment (MCI) and Alzheimer's Disease (AD). Although there is a strong interaction between motor and cognitive functions, intact speech motor control is a crucial yet often-overlooked component of cognitive functioning. Additionally, motor decline can occur independently and may precede the onset of cognitive impairment in neurodegenerative conditions. These impairments can confound measures of higher-order cognition, typically assessed through behavioral performance. Notably, the associations between cognitive performance and biological indices of speech motor production have been largely unexplored. This study is the first to examine cognitive associations of cortical thickness in brain regions implicated in speech motor performance across the adult lifespan, and to investigate whether age-related structural changes in speech motor regions precede those seen in the hippocampus. Our sample included 699 cognitively healthy adults (56% female) spanning 35-90 years from the Human Connectome Project (HCP)-Aging dataset. Cognition was estimated using standard neuropsychological assessments including: the Trail Making Task A/B (TMT), the Rey Auditory Verbal Learning Test (RAVLT), and a cognitive composite score (summating cognitive performance across multiple tasks). Whole-brain T1- and T2-weighted MRI images were acquired using 3-Tesla scanners across multiple study sites. Structural images were preprocessed using the HCP minimal preprocessed pipelines to reconstruct cortical surfaces. Volume-based estimates including hippocampal volume and total gray matter volume were adjusted for head size using an adjusted measure of estimated Total Intracranial Volume (eTIV). Speech motor regions were investigated relative to well-characterized relationships with hippocampal volume (a hallmark region for memory and cognition and AD-related atrophy). Estimates of cortical thickness were extracted from 14 bilateral speech motor control regions spanning premotor, motor, somatosensory, insular, and prefrontal cortices. Performance across all cognitive tasks and estimates of brain structure were all highly correlated with age. After controlling for the effects of age, greater hippocampal volume remained correlated with better cognitive performance across all cognitive tasks. However, only cognitive associations with greater total gray matter volume survived correction for multiple comparisons. As expected, age associations with hippocampal volume differed between early (-0.191%/year) and late adulthood (-0.714%/year) (T = 6.179, p = 0.0002). Age associations with speech motor control regions significantly differed from the associations seen in GMV, mCT, and/or hippocampal volume across the lifespan (Pcor < 0.0001) and during late adulthood when compared separately. Half the speech motor control regions explored showed decelerated estimated percent difference per year from early and late adulthood. Our results suggest that neurocognitive relationships are highly impacted and often confounded by age. The thickness of several speech motor regions was not associated with cognitive performance and can therefore provide a more intrinsic measure of aging. Additionally, speech motor control regions decline earlier in adulthood than hippocampal volume and may therefore serve as a target and early indicator of AD-related neurodegeneration. This nuanced understanding is critical for refining early diagnostic criteria for neurodegenerative diseases, including AD, and sheds light on the complex interplay between age-related changes, disease pathology, and cognitive decline.
© 2025 The Author(s). Human Brain Mapping published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Predicting cognitive decline: Deep-learning reveals subtle brain changes in pre-MCI stage.J Prev Alzheimers Dis. 2025 May;12(5):100079. doi: 10.1016/j.tjpad.2025.100079. Epub 2025 Feb 6. J Prev Alzheimers Dis. 2025. PMID: 39920001 Free PMC article.
-
Cross-Sectional Comparison of Structural MRI Markers of Impairment in a Diverse Cohort of Older Adults.Hum Brain Mapp. 2025 Feb 1;46(2):e70133. doi: 10.1002/hbm.70133. Hum Brain Mapp. 2025. PMID: 39868891 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Selegiline for Alzheimer's disease.Cochrane Database Syst Rev. 2003;(1):CD000442. doi: 10.1002/14651858.CD000442. Cochrane Database Syst Rev. 2003. PMID: 12535396
References
-
- Ackermann, H. , and Riecker A.. 2004. “The Contribution of the Insula to Motor Aspects of Speech Production: A Review and a Hypothesis.” Brain and Language 89, no. 2: 320–328. - PubMed
-
- Ackermann, H. , and Riecker A.. 2010. “The Contribution (s) of the Insula to Speech Production: A Review of the Clinical and Functional Imaging Literature.” Brain Structure and Function 214: 419–433. - PubMed
-
- Aljondi, R. , Szoeke C., Steward C., Yates P., and Desmond P.. 2019. “A Decade of Changes in Brain Volume and Cognition.” Brain Imaging and Behavior 13: 554–563. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

