Surface-Modified Nanozymes for Enhanced and Selective Catalysis
- PMID: 40698926
- PMCID: PMC12332837
- DOI: 10.1021/acsami.5c07647
Surface-Modified Nanozymes for Enhanced and Selective Catalysis
Abstract
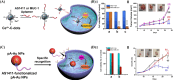
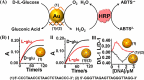
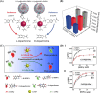
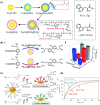
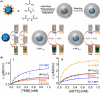
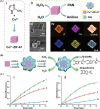
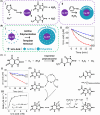
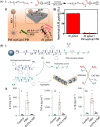
Surface-modified catalytic nanoparticles (nanozymes) are introduced as hybrid nanoparticles overcoming basic limitations associated with bare nanozymes that include moderate catalytic turnovers, lack of substrate selectivity and chiroselectivity, and poor or nonselective permeabilities into biomembrane. This review introduces aptamer-modified nanozymes, receptor (cyclodextrins)- or ligand (amino acids, peptides)-functionalized catalytic nanoparticles, and molecularly imprinted polymer-coated nanozymes as hybrid frameworks improving the catalytic properties and selective/chiroselective functions of the nanozymes. Binding of the reaction substrates to the aptamers, ligands, or molecular-imprinted sites, by affinity interactions, concentrates the substrates in spatial proximity to the nanozyme catalytic sites ("molarity effect"), thereby enhancing the catalytic performance of the frameworks. Specific and chiroselective binding interactions of the substrates to the surface modifiers lead to selective or chiroselective chemical transformations. Moreover, by appropriate molecular engineering of the surface modifiers on the nanozymes, catalytic functions lacking in the parent bare nanozymes are demonstrated. Potential applications of surface-modified nanozymes are discussed.
Keywords: Aptamer; Chiroselectivity; Molecular-imprinted polymer; Nanomedicine; Nanoparticle; Reactive oxygen species (ROS).
Figures


Similar articles
-
Parallel comparative studies on composition-dependent peroxidase-like catalytic activity of ultrasmall ferrite nanoparticles.J Mater Chem B. 2025 Jul 16;13(28):8434-8445. doi: 10.1039/d5tb00626k. J Mater Chem B. 2025. PMID: 40531135
-
Strategies for regulating catalytic specificity of nanozyme.Colloids Surf B Biointerfaces. 2025 Jul 1;255:114925. doi: 10.1016/j.colsurfb.2025.114925. Online ahead of print. Colloids Surf B Biointerfaces. 2025. PMID: 40617141 Review.
-
Nanozymes: Classification and Analytical Applications - A Review.J Fluoresc. 2025 Jul;35(7):4973-4987. doi: 10.1007/s10895-024-03930-3. Epub 2024 Sep 13. J Fluoresc. 2025. PMID: 39271600 Review.
-
Nanozymes with biomimetically designed properties for cancer treatment.Nanoscale. 2024 Apr 25;16(16):7786-7824. doi: 10.1039/d4nr00155a. Nanoscale. 2024. PMID: 38568434 Review.
-
Design of novel hybrid probe based on double recognition of aptamer-molecularly imprinted polymer-gold nanoparticles for food allergen gliadin sensing.Talanta. 2025 Dec 1;295:128344. doi: 10.1016/j.talanta.2025.128344. Epub 2025 May 28. Talanta. 2025. PMID: 40441110
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

