Identification of novel human microcephaly-linked protein Mtss2 that mediates cortical progenitor cell division and corticogenesis through Nedd9-RhoA
- PMID: 40698928
- PMCID: PMC12286603
- DOI: 10.7554/eLife.92748
Identification of novel human microcephaly-linked protein Mtss2 that mediates cortical progenitor cell division and corticogenesis through Nedd9-RhoA
Abstract
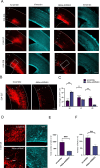
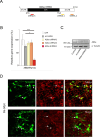
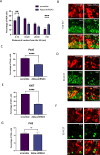
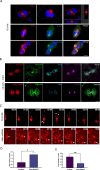
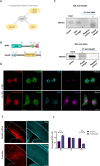
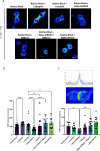
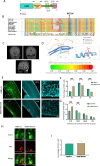
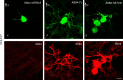
The cerebral cortex, which is responsible for higher cognitive functions, relies on the coordinated asymmetric division cycles of polarized radial glial progenitor cells for proper development. Defects in the mitotic process of neuronal stem cells have been linked to the underlying causes of microcephaly; however, the exact mechanisms involved are not fully understood. In this study, we present a new discovery regarding the role of the membrane-deforming cytoskeletal regulator protein called Mtss2 (also known as MTSS1L/ABBA) in cortical development. When Mtss2 was absent in the developing brain, it led to a halt in radial glial cell proliferation, disorganized radial fibers, and abnormal migration of neuronal progenitors. During cell division, Mtss2 localized to the cleavage furrow, where it recruited the scaffolding protein Nedd9 and positively influenced the activity of RhoA, a crucial regulator of cell division. Notably, we identified a variant of Mtss2 (R671W) in a patient with microcephaly and intellectual disability, further highlighting its significance. The introduction of this mutant Mtss2 protein in mice resulted in phenotypic similarities to the effects of Mtss2 knockdown. Overall, these findings offer valuable mechanistic insights into the development of microcephaly and the cerebral cortex by identifying Mtss2 as a novel regulator involved in ensuring the accurate progression of mitosis in neuronal progenitor cells.
Keywords: ABBA (MTSS1L/MTSS2); RhoA signaling; corticogenesis; human; microcephaly; mitosis; mouse; neuroscience; radial glial cells.
© 2024, Carabalona et al.
Conflict of interest statement
AC, HK, MG, LA, EE, FM, CF, PL, MW, JS, CR No competing interests declared
Figures




Update of
- doi: 10.1101/2022.03.28.22272122
- doi: 10.7554/eLife.92748.1
- doi: 10.7554/eLife.92748.2
- doi: 10.7554/eLife.92748.3
References
-
- Alazami AM, Patel N, Shamseldin HE, Anazi S, Al-Dosari MS, Alzahrani F, Hijazi H, Alshammari M, Aldahmesh MA, Salih MA, Faqeih E, Alhashem A, Bashiri FA, Al-Owain M, Kentab AY, Sogaty S, Al Tala S, Temsah M-H, Tulbah M, Aljelaify RF, Alshahwan SA, Seidahmed MZ, Alhadid AA, Aldhalaan H, AlQallaf F, Kurdi W, Alfadhel M, Babay Z, Alsogheer M, Kaya N, Al-Hassnan ZN, Abdel-Salam GMH, Al-Sannaa N, Al Mutairi F, El Khashab HY, Bohlega S, Jia X, Nguyen HC, Hammami R, Adly N, Mohamed JY, Abdulwahab F, Ibrahim N, Naim EA, Al-Younes B, Meyer BF, Hashem M, Shaheen R, Xiong Y, Abouelhoda M, Aldeeri AA, Monies DM, Alkuraya FS. Accelerating novel candidate gene discovery in neurogenetic disorders via whole-exome sequencing of prescreened multiplex consanguineous families. Cell Reports. 2015;10:148–161. doi: 10.1016/j.celrep.2014.12.015. - DOI - PubMed
-
- Carlson BR, Lloyd KE, Kruszewski A, Kim IH, Rodriguiz RM, Heindel C, Faytell M, Dudek SM, Wetsel WC, Soderling SH. WRP/srGAP3 facilitates the initiation of spine development by an inverse F-BAR domain, and its loss impairs long-term memory. The Journal of Neuroscience. 2011;31:2447–2460. doi: 10.1523/JNEUROSCI.4433-10.2011. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

