Randomized, Double-Blind, Placebo-Controlled, Multiple-Dose Studies to Assess the Safety and Efficacy of Elezanumab when Added to Standard of Care in Relapsing and Progressive Forms of Multiple Sclerosis
- PMID: 40698976
- PMCID: PMC12392050
- DOI: 10.1002/ana.27262
Randomized, Double-Blind, Placebo-Controlled, Multiple-Dose Studies to Assess the Safety and Efficacy of Elezanumab when Added to Standard of Care in Relapsing and Progressive Forms of Multiple Sclerosis
Abstract
Objective: Elezanumab is a monoclonal antibody that binds repulsive guidance molecule a (RGMa), an inhibitor of central nervous system regeneration after inflammation or injury. The aim was to assess the safety and efficacy of elezanumab in relapsing and progressive forms of multiple sclerosis (MS).
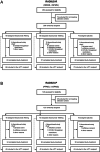
Methods: RADIUS-R and RADIUS-P were phase 2 trials in relapsing (RADIUS-R) or progressive (RADIUS-P) MS. Participants were randomized to intravenous elezanumab 400mg, 1800mg, or placebo every 4 weeks through week 48. The primary endpoint was the mean Overall Response Score (ORS).
Results: In RADIUS-R, 208 participants received elezanumab 400mg (n = 69), elezanumab 1800mg (n = 69), or placebo (n = 70). In RADIUS-P, 123 participants received elezanumab 400mg (n = 40), elezanumab 1800mg (n = 40), or placebo (n = 43). The primary endpoint of ORS was not met in either study. For RADIUS-R, mean ORS was -0.2 with effect size of -0.2 for elezanumab 400mg and -0.2 with effect size of -0.2 for elezanumab 1800mg. For RADIUS-P, mean ORS was 0.0 with effect size of 0.0 for elezanumab 400mg and 0.1 with effect size of 0.1 for elezanumab 1800mg. Elezanumab was well tolerated; the rate of serious adverse events was similar across treatment groups in both studies. Adverse events with ≥10% of elezanumab population were falls, urinary tract infections, headaches in RADIUS-R and RADIUS-P, and also fatigue, infusion-related reactions, and muscular weakness in RADIUS-P.
Interpretation: Elezanumab was safe and well tolerated, but did not meet the primary endpoint in either study. ANN NEUROL 2025;98:590-602.
© 2025 The Author(s). Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
B.A.C.C. and M.S.F. have nothing to report. M.G. and A.Z. disclose former employment and holdings of stock and/or stock options with AbbVie, which manufactures a drug used in the study. K.P. and B.S. disclose employment and stock and/or stock options with AbbVie, which manufactures a drug used in the study. A.W. discloses research support and compensation as an advisor from AbbVie, which manufactures a drug used in the study.
Figures


Similar articles
-
Azathioprine for people with multiple sclerosis.Cochrane Database Syst Rev. 2024 Dec 9;12(12):CD015005. doi: 10.1002/14651858.CD015005.pub2. Cochrane Database Syst Rev. 2024. PMID: 39651635 Free PMC article.
-
Natalizumab for relapsing remitting multiple sclerosis.Cochrane Database Syst Rev. 2011 Oct 5;(10):CD007621. doi: 10.1002/14651858.CD007621.pub2. Cochrane Database Syst Rev. 2011. PMID: 21975773
-
Teriflunomide for multiple sclerosis.Cochrane Database Syst Rev. 2016 Mar 22;3(3):CD009882. doi: 10.1002/14651858.CD009882.pub3. Cochrane Database Syst Rev. 2016. PMID: 27003123 Free PMC article.
-
Immunomodulators and immunosuppressants for multiple sclerosis: a network meta-analysis.Cochrane Database Syst Rev. 2013 Jun 6;2013(6):CD008933. doi: 10.1002/14651858.CD008933.pub2. Cochrane Database Syst Rev. 2013. PMID: 23744561 Free PMC article.
-
Rituximab for relapsing-remitting multiple sclerosis.Cochrane Database Syst Rev. 2013 Dec 6;2013(12):CD009130. doi: 10.1002/14651858.CD009130.pub3. Cochrane Database Syst Rev. 2013. PMID: 24310855 Free PMC article.
References
-
- Campbell JD, Ghushchyan V, Brett McQueen R, et al. Burden of multiple sclerosis on direct, indirect costs and quality of life: national US estimates. Mult Scler Relat Disord 2014;3:227–236. - PubMed
-
- Stahl B, Müller B, von Boxberg Y, et al. Biochemical characterization of a putative axonal guidance molecule of the chick visual system. Neuron 1990;5:735–743. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

